Recombinant Saccharomyces cerevisiae expressing P450 in artificial digestive systems: a model for biodetoxication in the human digestive environment
- PMID: 12732562
- PMCID: PMC154485
- DOI: 10.1128/AEM.69.5.2884-2892.2003
Recombinant Saccharomyces cerevisiae expressing P450 in artificial digestive systems: a model for biodetoxication in the human digestive environment
Abstract
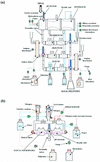
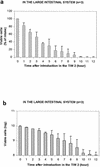
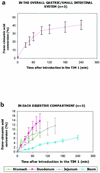
The use of genetically engineered microorganisms such as bacteria or yeasts as live vehicles to carry out bioconversion directly in the digestive environment is an important challenge for the development of innovative biodrugs. A system that mimics the human gastrointestinal tract was combined with a computer simulation to evaluate the survival rate and cinnamate 4-hydroxylase activity of a recombinant model of Saccharomyces cerevisiae expressing the plant P450 73A1. The yeasts showed a high level of resistance to gastric and small intestinal secretions (survival rate after 4 h of digestion, 95.6% +/- 10.1% [n = 4]) but were more sensitive to the colonic conditions (survival rate after 4 h of incubation, 35.9% +/- 2.7% [n = 3]). For the first time, the ability of recombinant S. cerevisiae to carry out a bioconversion reaction has been demonstrated throughout the gastrointestinal tract. In the gastric-small intestinal system, 41.0% +/- 5.8% (n = 3) of the ingested trans-cinnamic acid was converted into p-coumaric acid after 4 h of digestion, as well as 8.9% +/- 1.6% (n = 3) in the stomach, 13.8% +/- 3.3% (n = 3) in the duodenum, 11.8% +/- 3.4% (n = 3) in the jejunum, and 6.5% +/- 1.0% (n = 3) in the ileum. In the large intestinal system, cinnamate 4-hydroxylase activity was detected but was too weak to be quantified. These results suggest that S. cerevisiae may afford a useful host for the development of biodrugs and may provide an innovative system for the prevention or treatment of diseases that escape classical drug action. In particular, yeasts may provide a suitable vector for biodetoxication in the digestive environment.
Figures



Similar articles
-
Recombinant Saccharomyces cerevisiae strain expressing a model cytochrome P450 in the rat digestive environment: viability and bioconversion activity.Appl Environ Microbiol. 2007 Jun;73(11):3566-74. doi: 10.1128/AEM.02091-06. Epub 2007 Apr 6. Appl Environ Microbiol. 2007. PMID: 17416683 Free PMC article.
-
Effects of cryoprotectants on the viability and activity of freeze dried recombinant yeasts as novel oral drug delivery systems assessed by an artificial digestive system.Eur J Pharm Biopharm. 2005 Sep;61(1-2):32-9. doi: 10.1016/j.ejpb.2005.03.009. Eur J Pharm Biopharm. 2005. PMID: 16005198
-
Characterization of recombinant plant cinnamate 4-hydroxylase produced in yeast. Kinetic and spectral properties of the major plant P450 of the phenylpropanoid pathway.Eur J Biochem. 1994 Jun 15;222(3):843-50. doi: 10.1111/j.1432-1033.1994.tb18931.x. Eur J Biochem. 1994. PMID: 8026495
-
The 'biodrug' concept: an innovative approach to therapy.Trends Biotechnol. 2001 Oct;19(10):393-400. doi: 10.1016/S0167-7799(01)01739-5. Trends Biotechnol. 2001. PMID: 11587764 Review.
-
Engineered yeasts simulating P450-dependent metabolisms: tricks, myths and reality.Drug Metabol Drug Interact. 1994;11(3):169-200. doi: 10.1515/dmdi.1994.11.3.169. Drug Metabol Drug Interact. 1994. PMID: 12371439 Review. No abstract available.
Cited by
-
Development and validation of a continuous in vitro system reproducing some biotic and abiotic factors of the veal calf intestine.Appl Environ Microbiol. 2010 Aug;76(16):5592-600. doi: 10.1128/AEM.00524-10. Epub 2010 Jun 25. Appl Environ Microbiol. 2010. PMID: 20581176 Free PMC article.
-
Use of artificial digestive systems to investigate the biopharmaceutical factors influencing the survival of probiotic yeast during gastrointestinal transit in humans.Pharm Res. 2012 Jun;29(6):1444-53. doi: 10.1007/s11095-011-0620-5. Epub 2011 Nov 9. Pharm Res. 2012. PMID: 22068280
-
Cell engineering and molecular pharming for biopharmaceuticals.Open Med Chem J. 2008 May 14;2:49-61. doi: 10.2174/1874104500802010049. Open Med Chem J. 2008. PMID: 19662143 Free PMC article.
-
A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions.Pharm Res. 2004 Apr;21(4):585-91. doi: 10.1023/b:pham.0000022404.70478.4b. Pharm Res. 2004. PMID: 15139514
-
Development of a Potential Yeast-Based Vaccine Platform for Theileria parva Infection in Cattle.Front Immunol. 2021 Jul 8;12:674484. doi: 10.3389/fimmu.2021.674484. eCollection 2021. Front Immunol. 2021. PMID: 34305904 Free PMC article.
References
-
- Alric, M., S. Blanquet, S. Marol-Bonnin, D. Pompon, and M. Renaud. December 2000. Microorganismes actifs dans l'environnement digestif. International patent WO 01/98461.
-
- Bergogne-Berezin, E. 2000. Treatment and prevention of antibiotic associated diarrhoea. Int. J. Antimicrob. Agents 16:521-526. - PubMed
-
- Blanquet, S., S. Marol-Bonnin, E. Beyssac, D. Pompon, M. Renaud, and M. Alric. 2001. The biodrug concept: an innovative approach to therapy. Trends Biotechnol. 19:393-400. - PubMed
-
- Blehaut, H., J. Massot, G. W. Elmer, and R. Levy. 1989. Disposition kinetics of Saccharomyces boulardii in man and rat. Biopharm. Drug Dispos. 10:353-364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

