Oscillatory nucleocytoplasmic shuttling of the general stress response transcriptional activators Msn2 and Msn4 in Saccharomyces cerevisiae
- PMID: 12732613
- PMCID: PMC2172953
- DOI: 10.1083/jcb.200303030
Oscillatory nucleocytoplasmic shuttling of the general stress response transcriptional activators Msn2 and Msn4 in Saccharomyces cerevisiae
Abstract
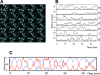
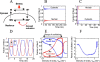
Msn2 and Msn4 are two related transcriptional activators that mediate a general response to stress in yeast Saccharomyces cerevisiae by eliciting the expression of specific sets of genes. In response to stress or nutritional limitation, Msn2 and Msn4 migrate from the cytoplasm to the nucleus. Using GFP-tagged constructs and high-resolution time-lapse video microscopy on single cells, we show that light emitted by the microscope also triggers this migration. Unexpectedly, the population of Msn2 or Msn4 molecules shuttles repetitively into and out of the nucleus with a periodicity of a few minutes. A large heterogeneity in the oscillatory response to stress is observed between individual cells. This periodic behavior, which can be induced by various types of stress, at intermediate stress levels, is not dependent upon protein synthesis and persists when the DNA-binding domain of Msn2 is removed. The cAMP-PKA pathway controls the sensitivity of the oscillatory nucleocytoplasmic shuttling. In the absence of PKA, Msn4 continues to oscillate while Msn2 is maintained in the nucleus. We show that a computational model based on the possibility that Msn2 and Msn4 participate in autoregulatory loops controlling their subcellular localization can account for the oscillatory behavior of the two transcription factors.
Figures




References
-
- Attfield, P.V., H.Y. Choi, D.A. Veal, and P.J. Bell. 2001. Heterogeneity of stress gene expression and stress resistance among individual cells of Saccharomyces cerevisiae. Mol. Microbiol. 40:1000–1008. - PubMed
-
- Belli, G., E. Gari, M. Aldea, and E. Herrero. 2001. Osmotic stress causes a G1 cell cycle delay and downregulation of Cln3/Cdc28 activity in Saccharomyces cerevisiae. Mol. Microbiol. 39:1022–1035. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

