A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase
- PMID: 12732615
- PMCID: PMC2172935
- DOI: 10.1083/jcb.200301105
A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase
Abstract
Centrosomes nucleate microtubules and contribute to mitotic spindle organization and function. They also participate in cytokinesis and cell cycle progression in ways that are poorly understood. Here we describe a novel human protein called centriolin that localizes to the maternal centriole and functions in both cytokinesis and cell cycle progression. Centriolin silencing induces cytokinesis failure by a novel mechanism whereby cells remain interconnected by long intercellular bridges. Most cells continue to cycle, reenter mitosis, and form multicellular syncytia. Some ultimately divide or undergo apoptosis specifically during the protracted period of cytokinesis. At later times, viable cells arrest in G1/G0. The cytokinesis activity is localized to a centriolin domain that shares homology with Nud1p and Cdc11p, budding and fission yeast proteins that anchor regulatory pathways involved in progression through the late stages of mitosis. The Nud1p-like domain of centriolin binds Bub2p, another component of the budding yeast pathway. We conclude that centriolin is required for a late stage of vertebrate cytokinesis, perhaps the final cell cleavage event, and plays a role in progression into S phase.
Figures
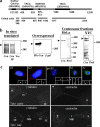
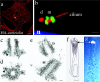


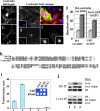
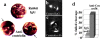

References
-
- Andersen, S.S. 2000. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 10:261–267. - PubMed
-
- Bardin, A.J., and A. Amon. 2001. Men and sin: what's the difference? Nat. Rev. Mol. Cell Biol. 2:815–826. - PubMed
-
- Blomberg-Wirschell, M., and S.J. Doxsey. 1998. Rapid isolation of centrosomes. Methods Enzymol. 298:228–238. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

