Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis
- PMID: 12732654
- PMCID: PMC2193967
- DOI: 10.1084/jem.20021603
Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis
Abstract
Multiple sclerosis (MS) is considered to be an autoimmune disease of the central nervous system (CNS) that in many patients first presents clinically as optic neuritis. The relationship of optic neuritis to MS is not well understood. We have generated novel T cell receptor (TCR) transgenic mice specific for myelin oligodendrocyte glycoprotein (MOG). MOG-specific transgenic T cells are not deleted nor tolerized and are functionally competent. A large proportion (>30%) of MOG-specific TCR transgenic mice spontaneously develop isolated optic neuritis without any clinical nor histological evidence of experimental autoimmune encephalomyelitis (EAE). Optic neuritis without EAE could also be induced in these mice by sensitization with suboptimal doses of MOG. The predilection of these mice to develop optic neuritis is associated with higher expression of MOG in the optic nerve than in the spinal cord. These results demonstrate that clinical manifestations of CNS autoimmune disease will vary depending on the identity of the target autoantigen and that MOG-specific T cell responses are involved in the genesis of isolated optic neuritis.
Figures




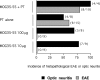
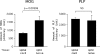
References
-
- Lassmann, H. 1998. Pathology of multiple sclerosis. McAlpine's Multiple Sclerosis. A. Compston, G. Ebers, H. Lassmann, I. McDonald, B. Matthews, and H. Wekerle, editors. Churchill Livingstone, Hong Kong. 323–358.
-
- Ghezzi, A., V. Martinelli, V. Torri, M. Zaffaroni, M. Rodegher, G. Comi, A. Zibetti, and N. Canal. 1999. Long-term follow-up of isolated optic neuritis: the risk of developing multiple sclerosis, its outcome, and the prognostic role of paraclinical tests. J. Neurol. 246:770–775. - PubMed
-
- Soderstrom, M. 2001. Optic neuritis and multiple sclerosis. Acta Ophthalmol. Scand. 79:223–227. - PubMed
-
- Zamvil, S.S., D.J. Mitchell, A.C. Moore, K. Kitamura, L. Steinman, and J.B. Rothbard. 1986. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 324:258–260. - PubMed
-
- Tuohy, V.K., Z. Lu, R.A. Sobel, R.A. Laursen, and M.B. Lees. 1989. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J. Immunol. 142:1523–1527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

