Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function
- PMID: 12732656
- PMCID: PMC2193970
- DOI: 10.1084/jem.20021910
Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function
Abstract
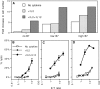
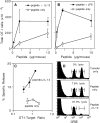
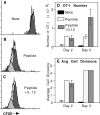
Activation of naive CD8 T cells to undergo clonal expansion and develop effector function requires three signals: (a) Ag, (b) costimulation, and (c) IL-12 or adjuvant. The requirement for the third signal to stimulate Ag-dependent proliferation is variable, making the greatest contribution when Ag levels are low. At high Ag levels, extensive proliferation can occur in vitro or in vivo in the absence of a third signal. However, despite having undergone the same number of divisions, cells that expand in the absence of a third signal fail to develop cytolytic effector function. Thus, proliferation and development of cytolytic function can be fully uncoupled. Furthermore, these cells are rendered functionally tolerant in vivo, in that subsequent restimulation with a potent stimulus results in limited clonal expansion, impaired IFN-gamma production, and no cytolytic function. Thus, the presence or absence of the third signal appears to be a critical variable in determining whether stimulation by Ag results in tolerance versus development of effector function and establishment of a responsive memory population.
Figures




References
-
- Allison, J., I.L. Campbell, G. Morahan, T.E. Mandel, L.C. Harrison, and J.F. Miller. 1988. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic beta cells. Nature. 333:529–533. - PubMed
-
- Burkly, L.C., D. Lo, O. Kanagawa, R.L. Brinster, and R.A. Flavell. 1989. T-cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 342:564–566. - PubMed
-
- Ferber, I., G. Schonrich, J. Schenkel, A. Mellor, G. Hammerling, and B. Arnold. 1994. Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science. 263:674–676. - PubMed
-
- Kyburz, D., P. Aichele, D. Speiser, H. Hengartner, R. Zinkernagel, and H. Pircher. 1993. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur. J. Immunol. 23:1956–1962. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

