Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons
- PMID: 12732711
- PMCID: PMC164529
- DOI: 10.1073/pnas.1137276100
Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons
Abstract
Kainate receptors (KA-Rs) are members of the glutamate-gated family of ionotropic receptors, which also includes N-methyl-d-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors. KA-Rs are important modulators of interneuron excitability in the CA1 region of the hippocampus. Activation of these receptors enhances interneuron firing, which robustly increases spontaneous inhibitory postsynaptic currents in pyramidal neurons. We report here that ethanol (EtOH) potently inhibits this KA-R-mediated effect at concentrations as low as those that can be achieved in blood after the ingestion of just 1-2 drinks (5-10 mM). Pressure application of kainate, in the presence of AMPA and NMDA receptor antagonists, evoked depolarizing responses in interneurons that triggered repetitive action potential firing. EtOH potently inhibited these responses to a degree that was sufficient to abolish action potential firing. This effect appears to be specific for KA-Rs, as EtOH did not affect action potential firing triggered by AMPA receptor-mediated depolarizing responses. Importantly, EtOH inhibited interneuron action potential firing in response to KA-R activation by synaptically released glutamate, suggesting that our findings are physiologically relevant. KA-R-dependent modulation of glutamate release onto pyramidal neurons was not affected by EtOH. Thus, EtOH increases excitability of pyramidal neurons indirectly by inhibiting the KA-R-dependent drive of gamma-aminobutyric acid (GABA)ergic interneurons. We postulate that this effect may explain, in part, some of the paradoxical excitatory actions of this widely abused substance. The excitatory actions of EtOH may be perceived as positive by some individuals, which could contribute to the development of alcoholism.
Figures


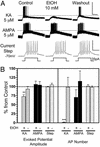

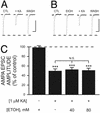

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

