Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice
- PMID: 12732718
- PMCID: PMC156288
- DOI: 10.1073/pnas.1036475100
Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice
Abstract
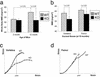
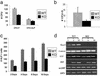
The cellular and molecular mechanisms that underlie age-dependent osteoporosis, the most common disease in the Western Hemisphere, are poorly understood in part due to the lack of appropriate animal models in which to study disease progression. Here, we present a model that shows many similarities to the human disease. Sca-1, well known for its expression on hematopoietic stem cells, is present on a subset of bone marrow stromal cells, which potentially include mesenchymal stem cells. Longitudinal studies showed that Sca-1(-/-) mice undergo normal bone development but with age exhibit dramatically decreased bone mass resulting in brittle bones. In vivo and in vitro analyses demonstrated that Sca-1 is required directly for the self-renewal of mesenchymal progenitors and indirectly for the regulation of osteoclast differentiation. Thus, defective mesenchymal stem or progenitor cell self-renewal may represent a previously uncharacterized mechanism of age-dependent osteoporosis in humans.
Figures



References
-
- Wasnich R D. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Favus M J, editor. Philadelphia: Lippincott Williams & Wilkins; 1996. pp. 249–251.
-
- Bellows C G, Wang Y H, Heersche J N M, Aubin J E. Endocrinology. 1994;134:2221–2229. - PubMed
-
- Aubin J E. J Cell Biochem. 1998;Suppl. 30/31:73–82. - PubMed
-
- Roodman G D. Exp Hematol. 1999;27:1229–1241. - PubMed
-
- Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

