Amyloid-beta peptide levels in brain are inversely correlated with insulysin activity levels in vivo
- PMID: 12732730
- PMCID: PMC156353
- DOI: 10.1073/pnas.1031520100
Amyloid-beta peptide levels in brain are inversely correlated with insulysin activity levels in vivo
Abstract
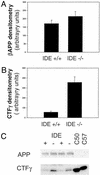
Factors that elevate amyloid-beta (Abeta) peptide levels are associated with an increased risk for Alzheimer's disease. Insulysin has been identified as one of several proteases potentially involved in Abeta degradation based on its hydrolysis of Abeta peptides in vitro. In this study, in vivo levels of brain Abeta40 and Abeta42 peptides were found to be increased significantly (1.6- and 1.4-fold, respectively) in an insulysin-deficient gene-trap mouse model. A 6-fold increase in the level of the gamma-secretase-generated C-terminal fragment of the Abeta precursor protein in the insulysin-deficient mouse also was found. In mice heterozygous for the insulysin gene trap, in which insulysin activity levels were decreased approximately 50%, brain Abeta peptides were increased to levels intermediate between those in wild-type mice and homozygous insulysin gene-trap mice that had no detectable insulysin activity. These findings indicate that there is an inverse correlation between in vivo insulysin activity levels and brain Abeta peptide levels and suggest that modulation of insulysin activity may alter the risk for Alzheimer's disease.
Figures



References
-
- Hardy J, Selkoe D J. Science. 2002;297:353–356. - PubMed
-
- Sambamurti K, Greig N H, Lahiri D K. Neuromol Med. 2002;1:1–31. - PubMed
-
- Kamal A, Almenar-Queralt A, LeBlanc J F, Roberts E A, Goldstein L S. Nature. 2001;414:643–648. - PubMed
-
- Nunan J, Small D H. FEBS Lett. 2000;483:6–10. - PubMed
-
- Kurochkin I V, Goto S. FEBS Lett. 1994;345:33–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

