Optimization of Naked DNA Delivery for Interferon Subtype Immunotherapy in Cytomegalovirus Infection
- PMID: 12734557
- PMCID: PMC150390
- DOI: 10.1251/bpo45
Optimization of Naked DNA Delivery for Interferon Subtype Immunotherapy in Cytomegalovirus Infection
Abstract
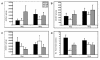
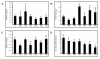
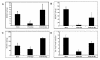
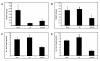
Type I interferon (IFN) gene therapy modulates the immune response leading to inflammatory heart disease following cytomegalovirus (CMV) infection in a murine model of post-viral myocarditis. Efficacy of different immunisation protocols for the IFN constructs was influenced by the dose of DNA, subtype choice, combination use, pre-medication, and timing of DNA administration. Optimal efficacy was found with bupivacaine treatment prior to DNA inoculation of 200mg IFN DNA 14 days prior to virus challenge. Maximal antiviral and antimyocarditic effects were achieved with this vaccination schedule. Furthermore, inoculation of synergistic IFN subtypes demonstrated enhanced efficacy when delivered either alone or with CMV gB DNA vaccination in the CMV model. Thus naked DNA delivery of IFN provides an avenue of immunotherapy for regulating herpesvirus-induced diseases.
Figures
References
-
- Gherardi MM, Ramirez JC, Esteban M. Interleukin-12 (IL-12) enhancement of the cellular immune response against human immunodeficiency virus type 1 Env antigen in a DNA prime/ vaccinia virus boost vaccine regimen is time and dose dependent: suppressive effects of IL-12 boost are mediated by nitric oxide. J Virol. 2000;74:6278–6286. doi: 10.1128/JVI.74.14.6278-6286.2000. - DOI - PMC - PubMed
-
- Prud’homme GJ, Lawson BR, Chang Y, Theofilopoulos AN. Immunotherapeutic gene transfer into muscle. Trends Immunol. 2001;22:149–155. - PubMed
LinkOut - more resources
Full Text Sources

