Sp1 and Sp3 are oxidative stress-inducible, antideath transcription factors in cortical neurons
- PMID: 12736330
- PMCID: PMC6742168
- DOI: 10.1523/JNEUROSCI.23-09-03597.2003
Sp1 and Sp3 are oxidative stress-inducible, antideath transcription factors in cortical neurons
Abstract
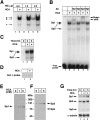
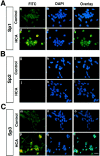
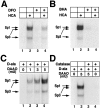
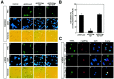
Neuronal cell death in response to oxidative stress may reflect the failure of endogenous adaptive mechanisms. However, the transcriptional activators induced by oxidative stress in neurons that trigger adaptive genetic responses have yet to be fully elucidated. We report that basal DNA binding of the zinc finger transcription factors Sp1 and Sp3 is unexpectedly low in cortical neurons in vitro and is significantly induced by glutathione depletion-induced or hydrogen peroxide-induced oxidative stress in these cells. The increases in Sp1/Sp3 DNA binding reflect, in part, increased levels of Sp1 and Sp3 protein in the nuclei of cortical neurons. Similar induction of Sp1 and Sp3 protein is also observed in neurons in vivo in a chemical or a genetic model of Huntington's disease, two rodent models in which neuronal loss has been attributed to oxidative stress. Sustained high-level expression of full-length Sp1 or full-length Sp3, but not the Sp1 zinc finger DNA-binding domain alone, prevents death in response to oxidative stress, DNA damage, or both. Taken together, these results establish Sp1 and Sp3 as oxidative stress-induced transcription factors in cortical neurons that positively regulate neuronal survival.
Figures



References
-
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm [Suppl] 2000;59:133–154. - PubMed
-
- Atwood CS, Huang X, Moir RD, Tanzi RE, Bush AI. Role of free radicals and metal ions in the pathogenesis of Alzheimer's disease. Met Ions Biol Syst. 1999;36:309–364. - PubMed
-
- Beal MF. Oxidative metabolism. Ann NY Acad Sci. 2000;924:164–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
