Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment
- PMID: 12736351
- PMCID: PMC6742177
- DOI: 10.1523/JNEUROSCI.23-09-03807.2003
Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment
Abstract
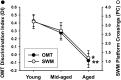
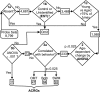
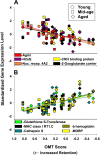
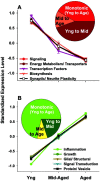
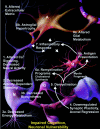
Gene expression microarrays provide a powerful new tool for studying complex processes such as brain aging. However, inferences from microarray data are often hindered by multiple comparisons, small sample sizes, and uncertain relationships to functional endpoints. Here we sought gene expression correlates of aging-dependent cognitive decline, using statistical profiling of gene microarrays in well powered groups of young, mid-aged, and aged rats (n = 10 per group). Animals were trained on two memory tasks, and the hippocampal CA1 region of each was analyzed on an individual microarray (one chip per animal). Aging- and cognition-related genes were identified by testing each gene by ANOVA (for aging effects) and then by Pearson's test (correlating expression with memory). Genes identified by this algorithm were associated with several phenomena known to be aging-dependent, including inflammation, oxidative stress, altered protein processing, and decreased mitochondrial function, but also with multiple processes not previously linked to functional brain aging. These novel processes included downregulated early response signaling, biosynthesis and activity-regulated synaptogenesis, and upregulated myelin turnover, cholesterol synthesis, lipid and monoamine metabolism, iron utilization, structural reorganization, and intracellular Ca2+ release pathways. Multiple transcriptional regulators and cytokines also were identified. Although most gene expression changes began by mid-life, cognition was not clearly impaired until late life. Collectively, these results suggest a new integrative model of brain aging in which genomic alterations in early adulthood initiate interacting cascades of decreased signaling and synaptic plasticity in neurons, extracellular changes, and increased myelin turnover-fueled inflammation in glia that cumulatively induce aging-related cognitive impairment.
Figures
References
-
- Austad SN. Why we age: what science is discovering about the body's journey through life. Wiley; Indianapolis: 1999.
-
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. - PubMed
-
- Becker KG (2002) Deciphering the gene expression profile of long-lived Snell mice. In: http://sageke.sciencemag.org - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
