Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase
- PMID: 12736361
- PMCID: PMC6742184
- DOI: 10.1523/JNEUROSCI.23-09-03916.2003
Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase
Abstract
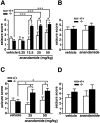
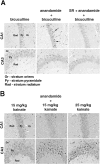
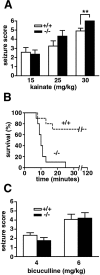
A number of recent in vitro studies have described a role for endogenous cannabinoids ("endocannabinoids") as transsynaptic modulators of neuronal activity in the hippocampus and other brain regions. However, the impact that endocannabinoid signals may have on activity-dependent neural events in vivo remains mostly unknown and technically challenging to address because of the short half-life of these chemical messengers in the brain. Mice lacking the enzyme fatty acid amide hydrolase [FAAH (-/-) mice] are severely impaired in their ability to degrade the endocannabinoid anandamide and therefore represent a unique animal model in which to examine the function of this signaling lipid in vivo. Here, we show that the administration of anandamide dramatically augments the severity of chemically induced seizures in FAAH (-/-) mice but not in wild-type mice. Anandamide-enhanced seizures in FAAH (-/-) mice resulted in significant neuronal damage in the CA1 and CA3 regions of the hippocampus for the bicuculline and kainate models, respectively. Notably, in the absence of anandamide treatment, FAAH (-/-) mice exhibited enhanced seizure responses to high doses of kainate that correlated with greatly elevated endogenous levels of anandamide in the hippocampus of these animals. Collectively, these studies suggest that both exogenously administered and endogenously produced anandamide display FAAH-regulated proconvulsant activity and do not support a general neuroprotective role for this endocannabinoid in response to excitotoxic stimuli in vivo. More generally, these findings demonstrate that the disinhibitory actions of endocannabinoids observed in hippocampal slices in vitro may also occur in vivo.
Figures

References
-
- Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–1684. - PubMed
-
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. - PubMed
-
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
