T lymphoid differentiation in human bone marrow
- PMID: 12738882
- PMCID: PMC164518
- DOI: 10.1073/pnas.1031503100
T lymphoid differentiation in human bone marrow
Abstract
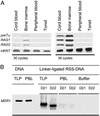
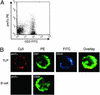
The unique role of the thymus in the development of T cells was established >4 decades ago. To elucidate how uncommitted lymphoid progenitor cells are instructed to migrate from bone marrow to the thymus to undergo T lymphoid differentiation, we generated and analyzed a genome-wide gene expression profile of CD7+ CD10+ human bone marrow T cell lineage precursors (TLPs) by using the serial analysis of gene expression technique. Unexpectedly, the serial analysis of gene expression profile identified a high number of (pre-) T cell receptor antigen (TCR)-related transcripts in bone marrow TLPs. To determine the configuration of the TCRbeta locus in these cells at a quantitative level, we sorted and analyzed bone marrow TLPs from five donors by single-cell PCR. Similar proportions of TLPs harbored TCRbeta germ-line alleles, D-J, or V-DJ gene rearrangements. Thus, bone marrow TLPs are heterogenous with respect to TCRbeta rearrangement status, suggesting an active recombination machinery that is consistent with the expression of RAG1, RAG2, and TdT in this population. As a hallmark of ongoing TCRbeta V-DJ rearrangement, we could amplify broken-ended recombination-signal sequence DNA intermediates from bone marrow TLPs, but not from mature T cells by ligation-mediated PCR. Approximately half of the TCRbeta rearrangements were compatible with the expression of a functional pre-TCR, which is in agreement with surface expression of pre-Talpha on bone marrow TLPs as shown by confocal laser microscopy and flow cytometry. At a frequency <0.5% of mononucleated cells in human bone marrow, this population is rare, yet it exemplifies T lymphoid differentiation in the human already before immigration into the thymus.
Figures

References
-
- Miller, J. F. A. P. (1961) Lancet ii, 748-749. - PubMed
-
- Markert, M. L., Boeck, A., Hale, L. P., Kloster, A. L., McLaughlin, T. M., Batchvarova, M. N., Douek, D. C., Koup, R. A., Kostyu, D. D., Ward, F. E., et al. (1999) N. Engl. J. Med. 341, 1180-1189. - PubMed
-
- Spits, H. (2002) Nat. Rev. Immunol. 2, 760-772. - PubMed
-
- Blom, B., Res, P., Noteboom, E., Weijer, K. & Spits, H. (1997) J. Immunol. 158, 3571-3577. - PubMed
-
- Deibakhsh-Jones, S., Jerabek, L., Weissman, I. L. & Strober, S. (1995) J. Immunol. 155, 3338-3344. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

