Instability of GGL domain-containing RGS proteins in mice lacking the G protein beta-subunit Gbeta5
- PMID: 12738888
- PMCID: PMC164494
- DOI: 10.1073/pnas.0631825100
Instability of GGL domain-containing RGS proteins in mice lacking the G protein beta-subunit Gbeta5
Abstract
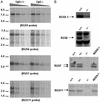
RGS (regulator of G protein signaling) proteins containing the G protein gamma-like (GGL) domain (RGS6, RGS7, RGS9, and RGS11) interact with the fifth member of the G protein beta-subunit family, Gbeta5. This interaction is necessary for the stability of both the RGS protein and for Gbeta5. Consistent with this notion, we have found that elevation of RGS9-1 mRNA levels by transgene expression does not increase RGS9-1 protein level in the retina, suggesting that Gbeta5 levels may be limiting. To examine further the interactions of Gbeta5 and the GGL domain-containing RGS proteins, we inactivated the Gbeta5 gene. We found that the levels of GGL domain-containing RGS proteins in retinas and in striatum are eliminated or reduced drastically, whereas the levels of Ggamma2 and RGS4 proteins remain normal in the absence of Gbeta5. The homozygous Gbeta5 knockout (Gbeta5-/-) mice derived from heterozygous knockout mating are runty and exhibit a high preweaning mortality rate. We concluded that complex formation between GGL domain-containing RGS proteins and the Gbeta5 protein is necessary to maintain their mutual stability in vivo. Furthermore, in the absence of Gbeta5 and all four RGS proteins that form protein complexes with Gbeta5, the animals that survive into adulthood are viable and have no gross defects in brain or retinal morphology.
Figures





References
-
- Koelle, M. R. & Horvitz, H. R. (1996) Cell 84, 115–125. - PubMed
-
- Berman, D. M., Wilkie, T. M. & Gilman, A. G. (1996) Cell 86, 445–452. - PubMed
-
- Ross, E. M. & Wilkie, T. M. (2000) Annu. Rev. Biochem. 69, 795–827. - PubMed
-
- Zheng, B., De Vries, L. & Gist Farquhar, M. (1999) Trends Biochem. Sci. 24, 411–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

