Septation and separation within the outflow tract of the developing heart
- PMID: 12739611
- PMCID: PMC1571094
- DOI: 10.1046/j.1469-7580.2003.00168.x
Septation and separation within the outflow tract of the developing heart
Abstract
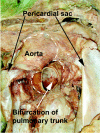
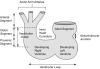
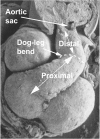
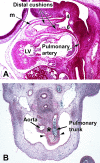
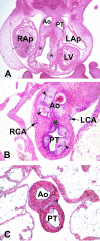
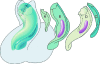
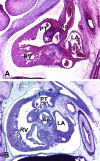
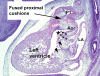
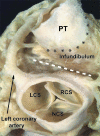
The developmental anatomy of the ventricular outlets and intrapericardial arterial trunks is a source of considerable confusion. First, major problems exist because of the multiple names and definitions used to describe this region of the heart as it develops. Second, there is no agreement on the boundaries of the described components, nor on the number of ridges or cushions to be found dividing the outflow tract, and the pattern of their fusion. Evidence is also lacking concerning the role of the fused cushions relative to that of the so-called aortopulmonary septum in separating the intrapericardial components of the great arterial trunks. In this review, we discuss the existing problems, as we see them, in the context of developmental and postnatal morphology. We concentrate, in particular, on the changes in the nature of the wall of the outflow tract, which is initially myocardial throughout its length. Key features that, thus far, do not seem to have received appropriate attention are the origin, and mode of separation, of the intrapericardial portions of the arterial trunks, and the formation of the walls of the aortic and pulmonary valvar sinuses. Also as yet undetermined is the formation of the free-standing muscular subpulmonary infundibulum, the mechanism of its separation from the aortic valvar sinuses, and its differentiation, if any, from the muscular ventricular outlet septum.
Figures

References
-
- Anderson RH. The anatomy of arteial valvar stenosis. Int. J. Cardiol. 1990;26:355–359. 10.1046/j.1469-7580.2003.00168.x. - DOI
-
- Anderson RH, Wilkinson JL, Arnold R, Lubkiewicz K. Morphogenesis of Bulboventricular malformations I: Consideration of embryogenesis in the normal heart. Br. Heart J. 1974a;36:2–42. 10.1046/j.1469-7580.2003.00168.x. - DOI - PMC - PubMed
-
- Anderson RH, Wilkinson JL, Arnold R, Becker AE, Lubkiewicz K. Morphogenesis of bulboventricular malformations II: observations on malformed hearts. Br. Heart J. 1974b;36:948–970. 10.1046/j.1469-7580.2003.00168.x. - DOI - PMC - PubMed
-
- Arguello C, De La Cruz MV, Sanchez C. Ultrastructural and experimental evidence of myocardial cell differentiation into connective tissue cells in embryonic chick heart. J. Mol. Cell Cardiol. 1978;10:307–315. 10.1046/j.1469-7580.2003.00168.x. - DOI - PubMed
-
- Arráez-Aybar La González-Lorrio F, Marantos-Gamarra Dg Jiménez-Collado J. Cardiac developmental onomatology: the real heart of the matter. J. Anat. 2003 in press. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

