Direct measurement of acid permeation into rat oesophagus
- PMID: 12740330
- PMCID: PMC1773668
- DOI: 10.1136/gut.52.6.775
Direct measurement of acid permeation into rat oesophagus
Abstract
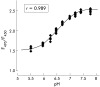
Background and aims: The early responses of the oesophageal mucosa to acid perfusion may predict subsequent pathology. Mucosal responses to luminal acid may result either from acid permeating through the mucosa or from other unknown transduction mechanisms. In order to better understand the dynamics of acid permeation into the oesophageal mucosa, we measured interstitial pH (pH(int)) of the oesophageal basal epithelial layer, pre-epithelial layer thickness, and blood flow in rats in vivo during luminal acid challenge. A novel confocal microscopic technique was used in vitro to measure pH(int) from defined cellular sites in response to luminal and basolateral acidification.
Methods: 5-(and-6)-Carboxyfluorescein (CF) and carboxy-seminapthorhodofluor-1 (SNARF-1) fluorescence was used to measure pH(int) by conventional and confocal microscopy, respectively, in urethane anaesthetised rats. Pre-epithelial layer thickness was measured optically with carbon particles as markers. Blood flow was measured with laser Doppler flowmetry.
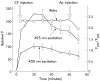
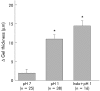
Results: Luminal acidification failed to alter pH(int) in vivo and in vitro, but pH(int) was lowered by modest serosal acidification. Pre-epithelial layer thickness and blood flow increased significantly during luminal surface acid perfusion. Indomethacin had no effect on any acid related response.
Conclusion: In this first dynamic measurement of oesophageal acid permeation and pre-epithelial layer thickness, pH(int) was preserved in spite of high luminal acidity by two complementary techniques. Despite the apparent permeability barrier to acid permeation, oesophageal blood flow and thickness responded to luminal acid perfusion.
Figures







References
-
- Fitzgerald RC, Omary MB, Triadafilopoulos G. Altered sodium-hydrogen exchange activity is a mechanism for acid-induced hyperproliferation in Barrett’s esophagus Am J Physiol Gastrointest Liver Physiol 1998;275:G47–55. - PubMed
-
- Bateson MC, Hopwood D, Milne G, et al. Oesophageal epithelial ultrastructure after incubation with gastrointestinal fluids and their components. J Pathol 1981;133:33–51. - PubMed
-
- Salo JA, Lehto VP, Kivilaakso E. Morphological alterations in experimental esophagitis. Light microscopic and scanning and transmission electron microscopic study. Dig Dis Sci 1983;28:440–8. - PubMed
-
- Sarosiek J, McCallum RW. Mechanisms of oesophageal mucosal defence. Baillieres Best Pract Res Clin Gastroenterol 14:701–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
