Gastrin activates nuclear factor kappaB (NFkappaB) through a protein kinase C dependent pathway involving NFkappaB inducing kinase, inhibitor kappaB (IkappaB) kinase, and tumour necrosis factor receptor associated factor 6 (TRAF6) in MKN-28 cells transfected with gastrin receptor
- PMID: 12740336
- PMCID: PMC1773663
- DOI: 10.1136/gut.52.6.813
Gastrin activates nuclear factor kappaB (NFkappaB) through a protein kinase C dependent pathway involving NFkappaB inducing kinase, inhibitor kappaB (IkappaB) kinase, and tumour necrosis factor receptor associated factor 6 (TRAF6) in MKN-28 cells transfected with gastrin receptor
Abstract
Background: We previously reported that gastrin induces expression of CXC chemokines through activation of nuclear factor kappaB (NFkappaB) in gastric epithelial cells that express gastrin receptor.
Aims: To clarify gastrin receptor mediated signals leading to activation of NFkappaB.
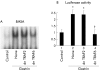
Methods: MKGR26 cells were created by transfecting gastrin receptor cDNA into MKN-28 cells. Degradation of inhibitor kappaB (IkappaB) and phosphorylation of protein kinase C (PKC)-delta were both detected by western blot analysis. NFkappaB activation was determined by luciferase assay and electrophoretic mobility shift analysis.
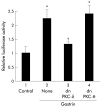
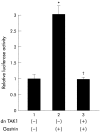
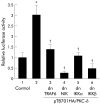
Results: Gastrin induced degradation of IkappaB-alpha and activation of NFkappaB, which was abolished by the selective gastrin receptor antagonist L-740,093 and the general PKC inhibitor GF109203X. Gastrin induced phosphorylation of PKC-delta, and its inhibitor rottlerin partially suppressed NFkappaB activation. However, the mitogen activated protein kinase (MAPK) kinase inhibitor PD98059, p38 MAPK inhibitor SB203580, and tyrphostin AG1478 had no effect on NFkappaB activation. Introduction of the dominant negative mutant of IkappaB kinase, of NFkappaB inducing kinase, and of tumour necrosis factor receptor associated factor 6 (TRAF6), but not that of TRAF2, inhibited gastrin induced activation of NFkappaB.
Conclusions: Gastrin activates NFkappaB via a PKC dependent pathway which involves IkappaB kinase, NFkappaB inducing kinase, and TRAF6.
Figures
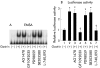
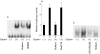

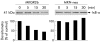

Similar articles
-
H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells.Gastroenterology. 2000 Jul;119(1):97-108. doi: 10.1053/gast.2000.8540. Gastroenterology. 2000. PMID: 10889159
-
Gastrin induces CXC chemokine expression in gastric epithelial cells through activation of NF-kappaB.Am J Physiol Gastrointest Liver Physiol. 2001 Sep;281(3):G735-42. doi: 10.1152/ajpgi.2001.281.3.G735. Am J Physiol Gastrointest Liver Physiol. 2001. PMID: 11518686
-
Gastrin induces heparin-binding epidermal growth factor-like growth factor in rat gastric epithelial cells transfected with gastrin receptor.Gastroenterology. 1999 Jan;116(1):78-89. doi: 10.1016/s0016-5085(99)70231-3. Gastroenterology. 1999. PMID: 9869605
-
Intracellular signaling in rat cultured vascular smooth muscle cells: roles of nuclear factor-kappaB and p38 mitogen-activated protein kinase on tumor necrosis factor-alpha production.Endocrinology. 1999 Aug;140(8):3562-72. doi: 10.1210/endo.140.8.6914. Endocrinology. 1999. PMID: 10433212
-
Signaling pathways mediating gastrin's growth-promoting effects.Peptides. 1999;20(7):885-98. doi: 10.1016/s0196-9781(99)00077-7. Peptides. 1999. PMID: 10477091 Review.
Cited by
-
Blocking gastrin and CCK-B autocrine loop affects cell proliferation and apoptosis in vitro.Mol Cell Biochem. 2010 Oct;343(1-2):133-41. doi: 10.1007/s11010-010-0507-5. Epub 2010 Jun 18. Mol Cell Biochem. 2010. PMID: 20559691
-
Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function.Sci Rep. 2017 Jan 18;7:40820. doi: 10.1038/srep40820. Sci Rep. 2017. PMID: 28098206 Free PMC article.
-
By activating matrix metalloproteinase-7, shear stress promotes chondrosarcoma cell motility, invasion and lung colonization.Oncotarget. 2015 Apr 20;6(11):9140-59. doi: 10.18632/oncotarget.3274. Oncotarget. 2015. PMID: 25823818 Free PMC article.
-
Activation of NFkappaB represents the central event in the neoplastic progression associated with Barrett's esophagus: a possible link to the inflammation and overexpression of COX-2, PPARgamma and growth factors.Dig Dis Sci. 2004 Aug;49(7-8):1075-83. doi: 10.1023/b:ddas.0000037790.11724.70. Dig Dis Sci. 2004. PMID: 15387324
-
Gastrokine 1 inhibits gastrin-induced cell proliferation.Gastric Cancer. 2016 Apr;19(2):381-391. doi: 10.1007/s10120-015-0483-2. Epub 2015 Mar 10. Gastric Cancer. 2016. PMID: 25752269 Free PMC article.
References
-
- Hiraoka S, Miyazaki Y, Kitamura S, et al. Gastrin induced CXC chemokine expression in gastric epithelial cells through activation of NF-κB. Am J Physiol Gastrointest Liver Physiol 2001;281:G735–42. - PubMed
-
- Yasumoto K, Okamoto N, Mukaida N, et al. Tumor necrosis factor α and interferon γ synergisitically induce interleukin 8 production in a human gastric cell line through acting concurrently on AP-1 and NF-κB-like binding sites of the interleukin 8 gene. J Biol Chem 1992;267:22506–11. - PubMed
-
- Masamune A, Shimosegawa T, Masamune O, et al. Helicobacter pylori-dependent ceramide production may mediate increased interleukin 8 expression in human gastric cancer cell lines. Gastroenterology 1999;116:1330–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
