Liquid-vapor oscillations of water in hydrophobic nanopores
- PMID: 12740433
- PMCID: PMC165830
- DOI: 10.1073/pnas.1136844100
Liquid-vapor oscillations of water in hydrophobic nanopores
Abstract
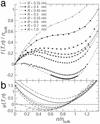
Water plays a key role in biological membrane transport. In ion channels and water-conducting pores (aquaporins), one-dimensional confinement in conjunction with strong surface effects changes the physical behavior of water. In molecular dynamics simulations of water in short (0.8 nm) hydrophobic pores the water density in the pore fluctuates on a nanosecond time scale. In long simulations (460 ns in total) at pore radii ranging from 0.35 to 1.0 nm we quantify the kinetics of oscillations between a liquid-filled and a vapor-filled pore. This behavior can be explained as capillary evaporation alternating with capillary condensation, driven by pressure fluctuations in the water outside the pore. The free-energy difference between the two states depends linearly on the radius. The free-energy landscape shows how a metastable liquid state gradually develops with increasing radius. For radii > approximately 0.55 nm it becomes the globally stable state and the vapor state vanishes. One-dimensional confinement affects the dynamic behavior of the water molecules and increases the self diffusion by a factor of 2-3 compared with bulk water. Permeabilities for the narrow pores are of the same order of magnitude as for biological water pores. Water flow is not continuous but occurs in bursts. Our results suggest that simulations aimed at collective phenomena such as hydrophobic effects may require simulation times >50 ns. For water in confined geometries, it is not possible to extrapolate from bulk or short time behavior to longer time scales.
Figures


Comment in
-
What happens if the room at the bottom runs out? A close look at small water pores.Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7421-2. doi: 10.1073/pnas.1533175100. Epub 2003 Jun 16. Proc Natl Acad Sci U S A. 2003. PMID: 12810943 Free PMC article. No abstract available.
References
-
- Doyle, D. A., Morais-Cabral, J., Pfützner, R. A., Kuo, A., Gulbis, J. M., Cohen, S. L., Chait, B. T. & MacKinnon, R. (1998) Science 280, 69-77. - PubMed
-
- Chang, G., Spencer, R. H., Lee, A. T., Barclay, M. T. & Rees, D. C. (1998) Science 282, 2220-2226. - PubMed
-
- Fu, D., Libson, A., Miercke, L. J., Weitzman, C., Nollert, P., Krucinski, J. & Stroud, R. M. (2000) Science 290, 481-486. - PubMed
-
- Sui, H., Han, B. G., Lee, J. K., Walian, P. & Jap, B. K. (2001) Nature 414, 872-878. - PubMed
-
- Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T. & MacKinnon, R. (2002) Nature 415, 287-294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

