Understanding tubulin-Taxol interactions: mutations that impart Taxol binding to yeast tubulin
- PMID: 12740436
- PMCID: PMC164457
- DOI: 10.1073/pnas.1131967100
Understanding tubulin-Taxol interactions: mutations that impart Taxol binding to yeast tubulin
Abstract
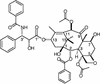
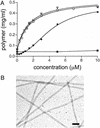
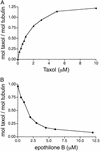
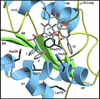
We have successfully used mutagenesis to engineer Taxol (paclitaxel) binding activity in Saccharomyces cerevisiae tubulin. Taxol, a successful antitumor agent, acts by promoting tubulin assembly and stabilizing microtubules. Several structurally diverse antimitotic compounds, including the epothilones, compete with Taxol for binding to mammalian microtubules, suggesting that Taxol and these compounds share an overlapping binding site. However, Taxol has no effect on tubulin or microtubules from S. cerevisiae, whereas epothilone does. After considering data on Taxol binding to mammalian tubulin and recent modeling studies, we have hypothesized that differences in five key amino acids are responsible for the lack of Taxol binding to yeast tubulin. After changing these amino acids to those found in mammalian brain tubulin, we observed Taxol-related activity in yeast tubulin comparable to that in mammalian tubulin. Importantly, this experimental system can be used to reveal tubulin interactions with Taxol, the epothilones, and other Taxol-like compounds.
Figures
References
-
- Jordan, M. A. (2002) Curr. Med. Chem. Anti-Cancer Agents 2, 1–17. - PubMed
-
- Jiménez-Barbero, J., Amat-Guerri, F. & Snyder, J. P. (2002) Curr. Med. Chem. Anti-Cancer Agents 2, 91–122. - PubMed
-
- Dasgupta, D., Park, H., Harriman, G. C., Georg, G. I. & Himes, R. H. (1994) J. Med. Chem. 37, 2976–2980. - PubMed
-
- Combeau, C., Commerçon, A., Mioskowski, C., Rousseau, B., Aubert, F. & Goeldner, M. (1994) Biochemistry 33, 6676–6683. - PubMed
-
- Rao, S., Krauss, N. E., Heerding, J. M., Swindell, C. S., Ringel, I., Orr, G. A. & Horwitz, S. B. (1994) J. Biol. Chem. 269, 3132–3134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

