Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family
- PMID: 12740605
- PMCID: PMC1319198
- DOI: 10.1038/sj.embor.embor848
Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family
Abstract
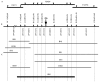
Ogura cytoplasmic male sterility (CMS) in radish (Raphanus sativus) is caused by an aberrant mitochondrial gene, Orf138, that prevents the production of functional pollen without affecting female fertility. Rfo, a nuclear gene that restores male fertility, alters the expression of Orf138 at the post-transcriptional level. The Ogura CMS/Rfo two-component system is a useful model for investigating nuclear-cytoplasmic interactions, as well as the physiological basis of fertility restoration. Using a combination of positional cloning and microsynteny analysis of Arabidopsis thaliana and radish, we genetically and physically delimited the Rfo locus to a 15-kb DNA segment. Analysis of this segment shows that Rfo is a member of the pentatricopeptide repeat (PPR) family. In Arabidopsis, this family contains more than 450 members of unknown function, although most of them are predicted to be targeted to mitochondria and chloroplasts and are thought to have roles in organellar gene expression.
Figures





References
-
- Aubourg S., Boudet N., Kreis M. & Lecharny A. (2000) In Arabidopsis thaliana, 1% of the genome codes for a novel protein family unique to plants. Plant Mol. Biol., 42, 603–613. - PubMed
-
- Barkan A. & Goldschmidt-Clermont M. (2000) Participation of nuclear genes in chloroplast gene expression. Biochimie, 82, 559–572. - PubMed
-
- Bendahmane A. Kanyuka K. & Baulcombe D.C. (1997) High-resolution genetical and physical mapping of the Rx gene for extreme resistance to potato virus X in tetraploid potato. Theor. Appl. Genet., 95, 153–162.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

