The integrin-actin connection, an eternal love affair
- PMID: 12743027
- PMCID: PMC156003
- DOI: 10.1093/emboj/cdg245
The integrin-actin connection, an eternal love affair
Abstract
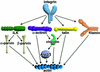
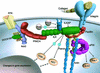
Integrin receptors connect the extracellular matrix to the actin cytoskeleton. This interaction can be viewed as a cyclical liaison, which develops again and again at new adhesion sites only to cease at sites of de-adhesion. Recent work has demonstrated that multidomain proteins play crucial roles in the integrin-actin connection by providing a high degree of regulation adjusted to the needs of the cell. In this review we present several examples of this paradigm and with special emphasis on the ILK-PINCH-parvin complex, which amply demonstrates how structural and signalling functions are linked together.
Figures

References
-
- Arthur W.T., Noren,N.K. and Burridge,K. (2002) Regulation of Rho family GTPases by cell–cell and cell–matrix adhesion. Biol. Res., 35, 239–246. - PubMed
-
- Aumailley M., Pesch,M., Tunggal,L., Gaill,F. and Fässler,R. (2000) Altered synthesis of laminin 1 and absence of basement membrane component deposition in β1 integrin-deficient embryoid bodies. J. Cell Sci., 113, 259–268. - PubMed
-
- Beggs A.H., Byers,T.J., Knoll,J.H., Boyce,F.M., Bruns,G.A. and Kunkel,L.M. (1992) Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J. Biol. Chem., 267, 9281–9288. - PubMed
-
- Bellanger J.M., Astier,C., Sardet,C., Ohta,Y., Stossel,T.P. and Debant,A. (2000) The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat. Cell Biol., 2, 888–892. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

