Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II
- PMID: 12743037
- PMCID: PMC156001
- DOI: 10.1093/emboj/cdg243
Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II
Abstract
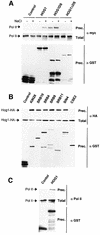
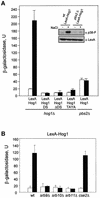
In budding yeast, the mitogen-activated protein kinase (MAPK) Hog1 coordinates the transcriptional program required for cell survival upon osmostress. The Hot1 transcription factor acts downstream of the MAPK and regulates a subset of Hog1-responsive genes. In response to high osmolarity, Hot1 targets Hog1 to specific osmostress-responsive promoters. Here, we show that assembly of the general transcription machinery at Hot1-dependent promoters depends on the presence of Hot1 and active Hog1 MAPK. Unexpectedly, recruitment of RNA polymerase (Pol) II complex to target promoters does not depend on the phosphorylation of the Hot1 activator by the MAPK. Hog1 interacts with the RNA Pol II and with general components of the transcription machinery. More over, when tethered to a promoter as a LexA fusion protein, Hog1 activates transcription in a stress- regulated manner. Thus, anchoring of active Hog1 to promoters by the Hot1 activator is essential for recruitment and activation of RNA Pol II. The mammalian p38 also interacts with the RNA Pol II, which might suggest a conserved mechanism for regulation of gene expression by SAPKs among eukaryotic cells.
Figures







References
-
- Alepuz P.M., Jovanovic,A., Reiser,V. and Ammerer,G. (2001) Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol. Cell, 7, 767–777. - PubMed
-
- Cosma M.P., Panizza,S. and Nasmyth,K. (2001) Cdk1 triggers association of RNA polymerase to cell cycle promoters only after recruitment of the mediator by SBF. Mol. Cell, 7, 1213–1220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

