Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments
- PMID: 12743103
- PMCID: PMC2172950
- DOI: 10.1083/jcb.200303138
Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments
Abstract
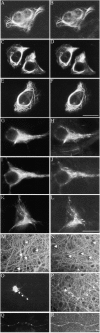
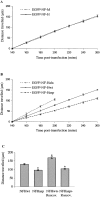
Neurofilaments possess side arms that comprise the carboxy-terminal domains of neurofilament middle and heavy chains (NFM and NFH); that of NFH is heavily phosphorylated in axons. Here, we demonstrate that phosphorylation of NFH side arms is a mechanism for regulating transport of neurofilaments through axons. Mutants in which known NFH phosphorylation sites were mutated to preclude phosphorylation or mimic permanent phosphorylation display altered rates of transport in a bulk transport assay. Similarly, application of roscovitine, an inhibitor of the NFH side arm kinase Cdk5/p35, accelerates neurofilament transport. Analyses of neurofilament movement in transfected living neurons demonstrated that a mutant mimicking permanent phosphorylation spent a higher proportion of time pausing than one that could not be phosphorylated. Thus, phosphorylation of NFH slows neurofilament transport, and this is due to increased pausing in neurofilament movement.
Figures




References
-
- Bajaj, N.P., and C.C.J. Miller. 1997. Phosphorylation of neurofilament heavy-chain side-arm fragments by cyclin-dependent kinase-5 and glycogen synthase kinase-3a in transfected cells. J. Neurochem. 69:737–743. - PubMed
-
- Brownlees, J., A. Yates, N.P. Bajaj, D. Davis, B.H. Anderton, P.N. Leigh, C.E. Shaw, and C.C.J. Miller. 2000. Phosphorylation of neurofilament heavy chain side-arms by stress activated protein kinase-1b/Jun N-terminal kinase-3. J. Cell Sci. 113:401–407. - PubMed
-
- Brownlees, J., S. Ackerley, A.J. Grierson, N.J. Jacobsen, K. Shea, B.H. Anderton, P.N. Leigh, C.E. Shaw, and C.C. Miller. 2002. Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum. Mol. Genet. 11:2837–2844. - PubMed
-
- Collard, J.-F., F. Cote, and J.-P. Julien. 1995. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 375:61–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

