Alpha-adducin dissociates from F-actin and spectrin during platelet activation
- PMID: 12743105
- PMCID: PMC2172952
- DOI: 10.1083/jcb.200211122
Alpha-adducin dissociates from F-actin and spectrin during platelet activation
Abstract
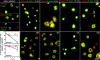
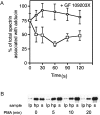
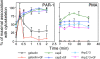
Aspectrin-based skeleton uniformly underlies and supports the plasma membrane of the resting platelet, but remodels and centralizes in the activated platelet. alpha-Adducin, a phosphoprotein that forms a ternary complex with F-actin and spectrin, is dephosphorylated and mostly bound to spectrin in the membrane skeleton of the resting platelet at sites where actin filaments attach to the ends of spectrin molecules. Platelets activated through protease-activated receptor 1, FcgammaRIIA, or by treatment with PMA phosphorylate adducin at Ser726. Phosphoadducin releases from the membrane skeleton concomitant with its dissociation from spectrin and actin. Inhibition of PKC blunts adducin phosphorylation and release from spectrin and actin, preventing the centralization of spectrin that normally follows cell activation. We conclude that adducin targets actin filament ends to spectrin to complete the assembly of the resting membrane skeleton. Dissociation of phosphoadducin releases spectrin from actin, facilitating centralization of spectrin, and leads to the exposure of barbed actin filament ends that may then participate in converting the resting platelet's disc shape into its active form.
Figures








References
-
- Amano, M., A. Chihara, K. Kimura, Y. Fukata, N. Nakamura, Y. Matsuura, and K. Kaibuchi. 1997. Formation of actin stress fibers and focal adhesions enhanced by rho-kinase. Science. 275:1308–1311. - PubMed
-
- Bennett, V., K. Gardner, and J.P. Steiner. 1988. Brain adducin: a protein kinase C substrate that may mediate site-directed assembly at the spectrin-actin junction. J. Biol. Chem. 263:5860–5869. - PubMed
-
- Falet, H., and F. Rendu. 1994. Calcium mobilisation controls tyrosine protein phosphorylation independently of the activation of protein kinase C in human platelets. FEBS Lett. 345:87–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

