deep-orange and carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster
- PMID: 12743107
- PMCID: PMC2172926
- DOI: 10.1083/jcb.200210166
deep-orange and carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster
Abstract
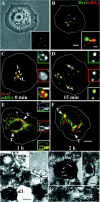
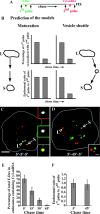
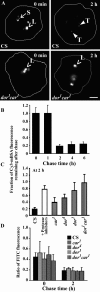
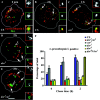
Endosomal degradation is severely impaired in primary hemocytes from larvae of eye color mutants of Drosophila. Using high resolution imaging and immunofluorescence microscopy in these cells, products of eye color genes, deep-orange (dor) and carnation (car), are localized to large multivesicular Rab7-positive late endosomes containing Golgi-derived enzymes. These structures mature into small sized Dor-negative, Car-positive structures, which subsequently fuse to form tubular lysosomes. Defective endosomal degradation in mutant alleles of dor results from a failure of Golgi-derived vesicles to fuse with morphologically arrested Rab7-positive large sized endosomes, which are, however, normally acidified and mature with wild-type kinetics. This locates the site of Dor function to fusion of Golgi-derived vesicles with the large Rab7-positive endocytic compartments. In contrast, endosomal degradation is not considerably affected in car1 mutant; fusion of Golgi-derived vesicles and maturation of large sized endosomes is normal. However, removal of Dor from small sized Car-positive endosomes is slowed, and subsequent fusion with tubular lysosomes is abolished. Overexpression of Dor in car1 mutant aggravates this defect, implicating Car in the removal of Dor from endosomes. This suggests that, in addition to an independent role in fusion with tubular lysosomes, the Sec1p homologue, Car, regulates Dor function.
Figures






Similar articles
-
A dominant negative form of the AAA ATPase SKD1/VPS4 impairs membrane trafficking out of endosomal/lysosomal compartments: class E vps phenotype in mammalian cells.J Cell Sci. 2003 Jan 15;116(Pt 2):401-14. doi: 10.1242/jcs.00213. J Cell Sci. 2003. PMID: 12482925
-
A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila.Mol Cell. 1999 Oct;4(4):479-86. doi: 10.1016/s1097-2765(00)80199-9. Mol Cell. 1999. PMID: 10549280
-
The SM protein Car/Vps33A regulates SNARE-mediated trafficking to lysosomes and lysosome-related organelles.Mol Biol Cell. 2009 Mar;20(6):1705-14. doi: 10.1091/mbc.e08-03-0282. Epub 2009 Jan 21. Mol Biol Cell. 2009. PMID: 19158398 Free PMC article.
-
How a RING finger protein and a steroid hormone control autophagy.Autophagy. 2006 Oct-Dec;2(4):321-2. doi: 10.4161/auto.3091. Epub 2006 Oct 14. Autophagy. 2006. PMID: 16874068 Review.
-
Lysosome-related organelles: unusual compartments become mainstream.Curr Opin Cell Biol. 2013 Aug;25(4):495-505. doi: 10.1016/j.ceb.2013.04.008. Epub 2013 May 29. Curr Opin Cell Biol. 2013. PMID: 23726022 Free PMC article. Review.
Cited by
-
The VPS33B-binding protein VPS16B is required in megakaryocyte and platelet α-granule biogenesis.Blood. 2012 Dec 13;120(25):5032-40. doi: 10.1182/blood-2012-05-431205. Epub 2012 Sep 21. Blood. 2012. PMID: 23002115 Free PMC article.
-
Sec20 is Required for Autophagic and Endocytic Degradation Independent of Golgi-ER Retrograde Transport.Cells. 2019 Jul 24;8(8):768. doi: 10.3390/cells8080768. Cells. 2019. PMID: 31344970 Free PMC article.
-
Accumulation of rhodopsin in late endosomes triggers photoreceptor cell degeneration.PLoS Genet. 2009 Feb;5(2):e1000377. doi: 10.1371/journal.pgen.1000377. Epub 2009 Feb 13. PLoS Genet. 2009. PMID: 19214218 Free PMC article.
-
Uptake of the necrotic serpin in Drosophila melanogaster via the lipophorin receptor-1.PLoS Genet. 2009 Jun;5(6):e1000532. doi: 10.1371/journal.pgen.1000532. Epub 2009 Jun 26. PLoS Genet. 2009. PMID: 19557185 Free PMC article.
-
Sphingosine kinases and their metabolites modulate endolysosomal trafficking in photoreceptors.J Cell Biol. 2011 Feb 21;192(4):557-67. doi: 10.1083/jcb.201004098. Epub 2011 Feb 14. J Cell Biol. 2011. PMID: 21321100 Free PMC article.
References
-
- Babst, M., G. Odorizzi, E.J. Estepa, and S.D. Emr. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 1:248–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

