Aberrant chemokine receptor expression and chemokine production by Langerhans cells underlies the pathogenesis of Langerhans cell histiocytosis
- PMID: 12743170
- PMCID: PMC2193776
- DOI: 10.1084/jem.20030137
Aberrant chemokine receptor expression and chemokine production by Langerhans cells underlies the pathogenesis of Langerhans cell histiocytosis
Abstract
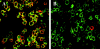
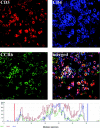
Langerhans cell histiocytosis (LCH) is characterized by a clonal proliferation and retention of cells with a Langerhans cell (LC)-like phenotype at various sites within the body. The present study set out to elucidate whether aberrant expression of chemokine receptors or dysregulation of chemokine production in LCH lesions could explain abnormal retention of these cells. Immunohistochemical analysis on 13 LCH biopsies of bone, skin, and lymph node all expressed the immature dendritic cell (DC) marker CCR6 on the lesional LCs and absence of the mature DC marker CCR7. Furthermore, regardless of the tissue site, LCH lesions markedly overexpressed CCL20/MIP-3alpha, the ligand for CCR6. The lesional LCs appeared to be the source of this CCL20/MIP-3alpha production as well as other inflammatory chemokines such as CCL5/RANTES and CXCL11/I-TAC. These may explain the recruitment of eosinophils and CD4+CD45RO+ T cells commonly found in LCH lesions. The findings of this study emphasize that, despite abundant TNF-alpha, lesional LCs remain in an immature state and are induced to produce chemokines, which via autocrine and paracrine mechanisms cause not only the retention of the lesional LCs but also the recruitment and retention of other lesional cells. We postulate that the lesional LCs themselves control the persistence and progression of LCH.
Figures


References
-
- Egeler, R.M., and G.J. D'Angio. 1995. Medical progress: Langerhans cell histiocytosis. J. Pediatr. 127:1–11. - PubMed
-
- Egeler, R.M., B.E. Favara, M. van Meurs, J.D. Laman, and E. Claassen. 1999. Differential in situ cytokine profiles of Langerhans-like cells and T cells in Langerhans cell histiocytosis: Abundant expression of cytokines relevant to disease and treatment. Blood. 94:4195–4201. - PubMed
-
- Greaves, D.R., W. Wang, D.J. Dairaghi, M.C. Dieu, B. Saint-Vis, K. Franz-Bacon, D. Rossi, C. Caux, T. McClanahan, S. Gordon, et al. 1997. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3α and is highly expressed in human dendritic cells. J. Exp. Med. 186:837–844. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

