Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells
- PMID: 12743275
- PMCID: PMC155023
- DOI: 10.1128/jvi.77.11.6188-6196.2003
Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells
Abstract
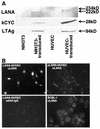
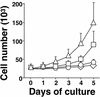
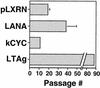
Tumor spindle cells in all clinical types of Kaposi's sarcoma (KS) are infected with Kaposi's sarcoma-associated herpesvirus (KSHV). Although KSHV contains more than 80 genes, only a few are expressed in tumor spindle cells, including latency-associated nuclear antigen (LANA) and k-cyclin (kCYC). To assess the oncogenic potential of LANA and kCYC, primary human umbilical vein endothelial cells (HUVEC) and murine NIH 3T3 cells were stably transduced by using recombinant retroviruses expressing these genes or the known viral oncogene simian virus 40 large T antigen (LTAg). Interestingly, LANA-transduced HUVEC proliferated faster and demonstrated a greatly prolonged life span (mean +/- standard deviation, 38.3 +/- 11.0 passages) than untransduced cells and vector-transduced cells (<20 passages). By contrast, kCYC-transduced HUVEC did not proliferate faster or live longer than control cells. LANA- and kCYC-transduced HUVEC, but not LTAg-transduced HUVEC, retained the ability to form normal vessel-like structures in an in vitro model of angiogenesis. In cellular assays of transformation, LANA- and kCYC-transduced NIH 3T3 cells demonstrated minimal or no anchorage-independent growth in soft agar and no tumorigenicity when injected into nude mice, unlike LTAg-transduced NIH 3T3 cells. Lastly, gene expression profiling revealed down-regulation, or silencing, of a number of genes within LANA-transduced HUVEC. Taken together, these results suggest that KSHV LANA is capable of inducing prolonged life span, but not transformation, in primary human cells. These findings may explain why LANA-expressing spindle cells proliferate within KS tumors, yet most often do not demonstrate biologic characteristics of transformation or true malignant conversion.
Figures



References
-
- Ascherl, G., C. Hohenadl, P. Monini, C. Zietz, P. J. Browning, B. Ensoli, and M. Sturzl. 1999. Expression of human herpesvirus-8 (HHV-8) encoded pathogenic genes in Kaposi's sarcoma (KS) primary lesions. Adv. Enzyme Regul. 39:331-339. - PubMed
-
- Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gerhengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. - PubMed
-
- Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency- associated nuclear antigen. Science 284:641-644. - PubMed
-
- Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. D. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial cells and spindle cells. Nat. Med. 1:1274-1278. - PubMed
-
- Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 90:8033-8037. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

