Transport of the intracisternal A-type particle Gag polyprotein to the endoplasmic reticulum is mediated by the signal recognition particle
- PMID: 12743286
- PMCID: PMC154983
- DOI: 10.1128/jvi.77.11.6293-6304.2003
Transport of the intracisternal A-type particle Gag polyprotein to the endoplasmic reticulum is mediated by the signal recognition particle
Abstract
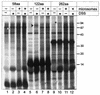
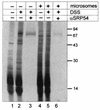
Intracisternal A-type particles (IAP) are defective endogenous retroviruses that accumulate in the endoplasmic reticulum (ER) of rodent cells. The enveloped particles are produced by assembly and budding of IAP Gag polyproteins at the ER membrane. In this study, we analyzed the specific ER transport of the Gag polyprotein of the IAP element MIA14. To this end, we performed in vitro translation of Gag in the presence of microsomal membranes or synthetic proteoliposomes followed by membrane sedimentation or flotation. ER binding of IAP Gag occurred mostly cotranslationally, and Gag polyproteins interacted specifically with proteoliposomes containing only signal recognition particle (SRP) receptor and the Sec61p complex, which form the minimal ER translocation apparatus. The direct participation of SRP in ER targeting of IAP Gag was demonstrated in cross-linking and immunoprecipitation experiments. The IAP polyprotein was not translocated into the ER; it was found to be tightly associated with the cytoplasmic side of the ER membrane but did not behave as an integral membrane protein. Substituting the functional signal peptide of preprolactin for the hydrophobic sequence at the N terminus of IAP Gag also did not result in translocation of the chimeric protein into the ER lumen, and grafting the IAP hydrophobic sequence onto preprolactin failed to yield luminal transport as well. These results suggest that the N-terminal hydrophobic region of the IAP Gag polyprotein functions as a transport signal which mediates SRP-dependent ER targeting, but polyprotein translocation or integration into the membrane is prevented by the signal sequence itself and by additional regions of Gag.
Figures






Similar articles
-
The beta subunit of the signal recognition particle receptor is a transmembrane GTPase that anchors the alpha subunit, a peripheral membrane GTPase, to the endoplasmic reticulum membrane.J Cell Biol. 1995 Feb;128(3):273-82. doi: 10.1083/jcb.128.3.273. J Cell Biol. 1995. PMID: 7844142 Free PMC article.
-
The hydrophobic region of signal peptides is a determinant for SRP recognition and protein translocation across the ER membrane.J Biochem. 1997 Feb;121(2):270-7. doi: 10.1093/oxfordjournals.jbchem.a021583. J Biochem. 1997. PMID: 9089400
-
The code for directing proteins for translocation across ER membrane: SRP cotranslationally recognizes specific features of a signal sequence.J Mol Biol. 2015 Mar 27;427(6 Pt A):1191-201. doi: 10.1016/j.jmb.2014.06.014. Epub 2014 Jun 28. J Mol Biol. 2015. PMID: 24979680 Free PMC article.
-
A second signal recognition event required for translocation into the endoplasmic reticulum.Cell. 1995 Jul 28;82(2):167-70. doi: 10.1016/0092-8674(95)90301-1. Cell. 1995. PMID: 7628005 Review.
-
Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane.Harvey Lect. 1995-1996;91:115-31. Harvey Lect. 1995. PMID: 9127989 Review. No abstract available.
Cited by
-
An infectious progenitor for the murine IAP retrotransposon: emergence of an intracellular genetic parasite from an ancient retrovirus.Genome Res. 2008 Apr;18(4):597-609. doi: 10.1101/gr.073486.107. Epub 2008 Feb 6. Genome Res. 2008. PMID: 18256233 Free PMC article.
-
Co-option of endogenous retroviruses through genetic escape from TRIM28 repression.Cell Rep. 2023 Jun 27;42(6):112625. doi: 10.1016/j.celrep.2023.112625. Epub 2023 Jun 7. Cell Rep. 2023. PMID: 37294634 Free PMC article.
-
The GLN family of murine endogenous retroviruses contains an element competent for infectious viral particle formation.J Virol. 2008 May;82(9):4413-9. doi: 10.1128/JVI.02141-07. Epub 2008 Feb 20. J Virol. 2008. PMID: 18287236 Free PMC article.
-
Murine MusD retrotransposon: structure and molecular evolution of an "intracellularized" retrovirus.J Virol. 2007 Feb;81(4):1888-98. doi: 10.1128/JVI.02051-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151128 Free PMC article.
-
P bodies inhibit retrotransposition of endogenous intracisternal a particles.J Virol. 2011 Jul;85(13):6244-51. doi: 10.1128/JVI.02517-10. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525359 Free PMC article.
References
-
- Anderson, D. J., K. E. Mostov, and G. Blobel. 1983. Mechanisms of integration of de novo-synthesized polypeptides into membranes: signal-recognition particle is required for integration into microsomal membranes of calcium ATPase and of lens MP26 but not of cytochrome b5. Proc. Natl. Acad. Sci. USA 80:7249-7253. - PMC - PubMed
-
- Aota, S., T. Gojobori, K. Shigesada, H. Ozeki, and T. Ikemura. 1987. Nucleotide sequence and molecular evolution of mouse retrovirus-like IAP elements. Gene 56:1-12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

