Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene
- PMID: 12743287
- PMCID: PMC154996
- DOI: 10.1128/jvi.77.11.6305-6313.2003
Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene
Abstract
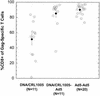
Cellular immune responses, particularly those associated with CD3(+) CD8(+) cytotoxic T lymphocytes (CTL), play a primary role in controlling viral infection, including persistent infection with human immunodeficiency virus type 1 (HIV-1). Accordingly, recent HIV-1 vaccine research efforts have focused on establishing the optimal means of eliciting such antiviral CTL immune responses. We evaluated several DNA vaccine formulations, a modified vaccinia virus Ankara vector, and a replication-defective adenovirus serotype 5 (Ad5) vector, each expressing the same codon-optimized HIV-1 gag gene for immunogenicity in rhesus monkeys. The DNA vaccines were formulated with and without one of two chemical adjuvants (aluminum phosphate and CRL1005). The Ad5-gag vector was the most effective in eliciting anti-Gag CTL. The vaccine produced both CD4(+) and CD8(+) T-cell responses, with the latter consistently being the dominant component. To determine the effect of existing antiadenovirus immunity on Ad5-gag-induced immune responses, monkeys were exposed to adenovirus subtype 5 that did not encode antigen prior to immunization with Ad5-gag. The resulting anti-Gag T-cell responses were attenuated but not abolished. Regimens that involved priming with different DNA vaccine formulations followed by boosting with the adenovirus vector were also compared. Of the formulations tested, the DNA-CRL1005 vaccine primed T-cell responses most effectively and provided the best overall immune responses after boosting with Ad5-gag. These results are suggestive of an immunization strategy for humans that are centered on use of the adenovirus vector and in which existing adenovirus immunity may be overcome by combined immunization with adjuvanted DNA and adenovirus vector boosting.
Figures
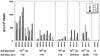






References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. - PMC - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
-
- Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

