Structure of intracellular mature vaccinia virus visualized by in situ atomic force microscopy
- PMID: 12743290
- PMCID: PMC155008
- DOI: 10.1128/jvi.77.11.6332-6340.2003
Structure of intracellular mature vaccinia virus visualized by in situ atomic force microscopy
Abstract
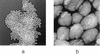
Vaccinia virus, the basis of the smallpox vaccine, is one of the largest viruses to replicate in humans. We have used in situ atomic force microscopy (AFM) to directly visualize fully hydrated, intact intracellular mature vaccinia virus (IMV) virions and chemical and enzymatic treatment products thereof. The latter included virion cores, core-enveloping coats, and core substructures. The isolated coats appeared to be composed of a highly cross-linked protein array. AFM imaging of core substructures indicated association of the linear viral DNA genome with a segmented protein sheath forming an extended approximately 16-nm-diameter filament with helical surface topography; enclosure of this filament within a 30- to 40-nm-diameter tubule which also shows helical topography; and enclosure of the folded, condensed 30- to 40-nm-diameter tubule within the core by a wall covered with peg-like projections. Proteins observed attached to the 30- to 40-nm-diameter tubules may mediate folding and/or compaction of the tubules and/or represent vestiges of the core wall and/or pegs. An accessory "satellite domain" was observed protruding from the intact core. This corresponded in size to isolated 70- to 100-nm-diameter particles that were imaged independently and might represent detached accessory domains. AFM imaging of intact virions indicated that IMV underwent a reversible shrinkage upon dehydration (as much as 2.2- to 2.5-fold in the height dimension), accompanied by topological and topographical changes, including protrusion of the satellite domain. As shown here, the chemical and enzymatic dissection of large, asymmetrical virus particles in combination with in situ AFM provides an informative complement to other structure determination techniques.
Figures







References
-
- Dales, S., and B. G. T. Pogo. 1981. Biology of poxviruses. Virology monographs. Springer-Verlag, Vienna, Austria. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

