Activation of fusion by the SER virus F protein: a low-pH-dependent paramyxovirus entry process
- PMID: 12743308
- PMCID: PMC155032
- DOI: 10.1128/jvi.77.11.6520-6527.2003
Activation of fusion by the SER virus F protein: a low-pH-dependent paramyxovirus entry process
Abstract
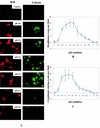
SER virus, a paramyxovirus closely related to simian virus 5, induces no syncytium formation. The SER virus F protein has a long cytoplasmic tail (CT), and truncation or mutations of the CT result in enhanced syncytium formation (S. Seth, A. Vincent, and R. W. Compans, J. Virol. 77:167-178, 2003; S. Tong, M. Li, A. Vincent, R. W. Compans, E. Fritsch, R. Beier, C. Klenk, M. Ohuchi, and H.-D. Klenk, Virology 301:322-333, 2002). We hypothesized that the presence of the long CT serves to stabilize the metastable conformation of the F protein. We observed that the hemifusion, cytoplasmic content mixing, and syncytium formation ability of the wild-type SER virus F coexpressed with the SER virus hemagglutinin-neuraminidase (HN) protein was enhanced, both qualitatively and quantitatively, at elevated temperatures. We also observed enhanced hemifusion, content mixing, and syncytium formation in SER virus F- and HN-expressing cells at reduced pH conditions ranging between 4.8 and 6.2. We have obtained evidence that in contrast to other paramyxoviruses, entry of SER virus into cells occurs by a low-pH-dependent process, indicating that the conversion to the fusion-active state for SER virus F is triggered by exposure to reduced pH.
Figures


Similar articles
-
Mutations in multiple domains activate paramyxovirus F protein-induced fusion.J Virol. 2004 Aug;78(16):8513-23. doi: 10.1128/JVI.78.16.8513-8523.2004. J Virol. 2004. PMID: 15280460 Free PMC article.
-
Mutations in the cytoplasmic domain of a paramyxovirus fusion glycoprotein rescue syncytium formation and eliminate the hemagglutinin-neuraminidase protein requirement for membrane fusion.J Virol. 2003 Jan;77(1):167-78. doi: 10.1128/jvi.77.1.167-178.2003. J Virol. 2003. PMID: 12477822 Free PMC article.
-
Analysis of the pH requirement for membrane fusion of different isolates of the paramyxovirus parainfluenza virus 5.J Virol. 2006 Mar;80(6):3071-7. doi: 10.1128/JVI.80.6.3071-3077.2006. J Virol. 2006. PMID: 16501116 Free PMC article.
-
Structure and function of a paramyxovirus fusion protein.Biochim Biophys Acta. 2003 Jul 11;1614(1):73-84. doi: 10.1016/s0005-2736(03)00164-0. Biochim Biophys Acta. 2003. PMID: 12873767 Review.
-
Determinants of organ tropism of sendai virus.Front Biosci. 1999 Oct 1;4:D642-5. doi: 10.2741/tashiro. Front Biosci. 1999. PMID: 10502551 Review.
Cited by
-
Sequential roles of receptor binding and low pH in forming prehairpin and hairpin conformations of a retroviral envelope glycoprotein.J Virol. 2004 Aug;78(15):8201-9. doi: 10.1128/JVI.78.15.8201-8209.2004. J Virol. 2004. PMID: 15254191 Free PMC article.
-
Effects of hemagglutinin-neuraminidase protein mutations on cell-cell fusion mediated by human parainfluenza type 2 virus.J Virol. 2008 Sep;82(17):8283-95. doi: 10.1128/JVI.00460-08. Epub 2008 Jun 18. J Virol. 2008. PMID: 18562539 Free PMC article.
-
Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation.Virology. 2010 Jan 20;396(2):226-37. doi: 10.1016/j.virol.2009.10.040. Epub 2009 Nov 18. Virology. 2010. PMID: 19922971 Free PMC article.
-
Mutations in multiple domains activate paramyxovirus F protein-induced fusion.J Virol. 2004 Aug;78(16):8513-23. doi: 10.1128/JVI.78.16.8513-8523.2004. J Virol. 2004. PMID: 15280460 Free PMC article.
-
Nanorobot Hardware Architecture for Medical Defense.Sensors (Basel). 2008 May 6;8(5):2932-2958. doi: 10.3390/s8052932. Sensors (Basel). 2008. PMID: 27879858 Free PMC article.
References
-
- Bizebard, T., B. Gigant, P. Rigolet, B. Rasmussen, O. Diat, P. Bosecke, S. A. Wharton, J. J. Skehel, and M. Knossow. 1995. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature 376:92-94. - PubMed
-
- Bullough, P. A., F. M. Hughson, A. C. Treharne, R. W. Ruigrok, J. J. Skehel, and D. C. Wiley. 1994. Crystals of a fragment of influenza haemagglutinin in the low pH induced conformation. J. Mol. Biol. 236:1262-1265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

