Effect of resiniferatoxin on the noxious heat threshold temperature in the rat: a novel heat allodynia model sensitive to analgesics
- PMID: 12746222
- PMCID: PMC1573831
- DOI: 10.1038/sj.bjp.0705234
Effect of resiniferatoxin on the noxious heat threshold temperature in the rat: a novel heat allodynia model sensitive to analgesics
Abstract
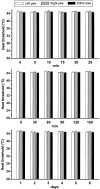
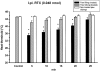
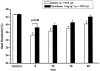
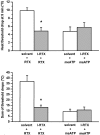
1. An increasing-temperature hot plate (ITHP) was introduced to measure the noxious heat threshold (45.3+/-0.3 degrees C) of unrestrained rats, which was reproducible upon repeated determinations at intervals of 5 or 30 min or 1 day. 2. Morphine, diclofenac and paracetamol caused an elevation of the noxious heat threshold following i.p. pretreatment, the minimum effective doses being 3, 10 and 200 mg kg(-1), respectively. 3. Unilateral intraplantar injection of the VR1 receptor agonist resiniferatoxin (RTX, 0.048 nmol) induced a profound drop of heat threshold to the innocuous range with a maximal effect (8-10 degrees C drop) 5 min after RTX administration. This heat allodynia was inhibited by pretreatment with morphine, diclofenac and paracetamol, the minimum effective doses being 1, 1 and 100 mg kg(-1) i.p., respectively. 4. The long-term sensory desensitizing effect of RTX was examined by bilateral intraplantar injection (0.048 nmol per paw) which produced, after an initial threshold drop, an elevation (up to 2.9+/-0.5 degrees C) of heat threshold lasting for 5 days. 5. The VR1 receptor antagonist iodo-resiniferatoxin (I-RTX, 0.05 nmol intraplantarly) inhibited by 51% the heat threshold-lowering effect of intraplantar RTX but not alpha,beta-methylene-ATP (0.3 micromol per paw). I-RTX (0.1 or 1 nmol per paw) failed to alter the heat threshold either acutely (5-60 min) or on the long-term (5 days). The heat threshold of VR1 receptor knockout mice was not different from that of wild-type animals (45.6+/-0.5 vs 45.2+/-0.4 degrees C). 6. In conclusion, the RTX-induced drop of heat threshold measured by the ITHP is a novel heat allodynia model exhibiting a high sensitivity to analgesics.
Figures



References
-
- AMABEOKU G.J., GREEN I., EAGLES P., BENJEDDOU M. Effects of Tarchonantus camphoratus and Eriocephalus africanus on nociception in mice and pyrexia in rats. Phytomedicine. 2000;7:517–522. - PubMed
-
- BITTNER M.A., LAHANN T.R. Biphasic time-course of capsaicin-induced substance P depletion: failure to correlate with thermal analgesia in the rat. Brain Res. 1984;322:305–309. - PubMed
-
- BJORKMAN R., HEDNER J., HEDNER T., HENNING M. Central, naloxone-reversible antinociception by diclofenac in the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:171–176. - PubMed
-
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. - PubMed
-
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

