Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling
- PMID: 12748279
- PMCID: PMC155228
- DOI: 10.1128/MCB.23.11.3753-3762.2003
Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling
Abstract
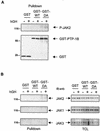
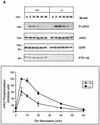
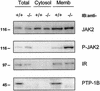
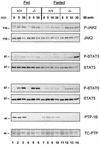
Protein tyrosine phosphatase-1B (PTP-1B) attenuates insulin, PDGF, EGF, and IGF-I signaling by dephosphorylating tyrosine residues located in the tyrosine kinase domain of the corresponding receptors. More recently, PTP-1B was shown to modulate the action of cytokine signaling via the nonreceptor tyrosine kinase JAK2. Transmission of the growth hormone (GH) signal also depends on JAK2, raising the possibility that PTP-1B modulates GH action. Consistent with this hypothesis, GH increased the abundance of tyrosine-phosphorylated JAK2 associated with a catalytically inactive mutant of PTP-1B. GH-induced JAK2 phosphorylation was greater in knockout (KO) than in wild-type (WT) PTP-1B embryonic fibroblasts and resulted in increased tyrosine phosphorylation of STAT3 and STAT5, while overexpression of PTP-1B reduced the GH-mediated activation of the acid-labile subunit gene. To evaluate the in vivo relevance of these observations, mice were injected with GH under fed and fasted conditions. As expected, tyrosine phosphorylation of JAK2 and STAT5 occurred readily in the livers of fed WT mice and was almost completely abolished during fasting. In contrast, resistance to the action of GH was severely impaired in the livers of fasted KO mice. These results indicate that PTP-1B regulates GH signaling by reducing the extent of JAK2 phosphorylation and suggest that PTP-1B is essential for limiting the action of GH during metabolic stress such as fasting.
Figures




Similar articles
-
Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase.Mol Endocrinol. 1996 Nov;10(11):1425-43. doi: 10.1210/mend.10.11.8923468. Mol Endocrinol. 1996. PMID: 8923468
-
Mapping of a cytoplasmic domain of the human growth hormone receptor that regulates rates of inactivation of Jak2 and Stat proteins.J Biol Chem. 1997 Apr 25;272(17):11128-32. doi: 10.1074/jbc.272.17.11128. J Biol Chem. 1997. PMID: 9111009
-
SOCS-3 is involved in the downregulation of the acute insulin-like effects of growth hormone in rat adipocytes by inhibition of Jak2/IRS-1 signaling.Horm Metab Res. 2003 Mar;35(3):169-77. doi: 10.1055/s-2003-39077. Horm Metab Res. 2003. PMID: 12734778
-
Growth hormone pulse-activated STAT5 signalling: a unique regulatory mechanism governing sexual dimorphism of liver gene expression.Novartis Found Symp. 2000;227:61-74; discussion 75-81. doi: 10.1002/0470870796.ch5. Novartis Found Symp. 2000. PMID: 10752065 Review.
-
SH2-B and SIRP: JAK2 binding proteins that modulate the actions of growth hormone.Recent Prog Horm Res. 2000;55:293-311. Recent Prog Horm Res. 2000. PMID: 11036942 Review.
Cited by
-
Scoparone interferes with STAT3-induced proliferation of vascular smooth muscle cells.Exp Mol Med. 2015 Mar 6;47(3):e145. doi: 10.1038/emm.2014.113. Exp Mol Med. 2015. PMID: 25744297 Free PMC article.
-
Protein tyrosine phosphatase 1B: a novel molecular target for retinal degenerative diseases.Adv Exp Med Biol. 2012;723:829-34. doi: 10.1007/978-1-4614-0631-0_106. Adv Exp Med Biol. 2012. PMID: 22183413 Free PMC article. Review.
-
Protein tyrosine phosphatase 1B participates in the down-regulation of erythropoietin receptor signalling.Biochem J. 2004 Jan 15;377(Pt 2):517-24. doi: 10.1042/BJ20031420. Biochem J. 2004. PMID: 14527337 Free PMC article.
-
Minecoside promotes apoptotic progression through STAT3 inactivation in breast cancer cells.Oncol Lett. 2022 Mar;23(3):94. doi: 10.3892/ol.2022.13214. Epub 2022 Jan 27. Oncol Lett. 2022. PMID: 35154425 Free PMC article.
-
Classical and novel GH receptor signaling pathways.Mol Cell Endocrinol. 2020 Dec 1;518:110999. doi: 10.1016/j.mce.2020.110999. Epub 2020 Aug 22. Mol Cell Endocrinol. 2020. PMID: 32835785 Free PMC article. Review.
References
-
- Aoki, N., and T. Matsuda. 2000. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J. Biol. Chem. 275:39718-39726. - PubMed
-
- Aoki, N., and T. Matsuda. 2002. A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus. Mol. Endocrinol. 16:58-69. - PubMed
-
- Argetsinger, L. S., G. S. Campbell, X. Yang, B. A. Witthuhn, O. Silvennoinen, J. N. Ihle, and C. Carter-Su. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237-244. - PubMed
-
- Beauloye, V., B. Willems, V. de Coninck, S. J. Frank, M. Edery, and J. P. Thissen. 2002. Impairment of liver GH receptor signaling by fasting. Endocrinology 143:792-800. - PubMed
-
- Boisclair, Y. R., J. Wang, J. Shi, K. R. Hurst, and G. T. Ooi. 2000. Role of the suppressor of cytokine signaling-3 in mediating the inhibitory effects of interleukin-1β on the growth hormone-dependent transcription of the acid-labile subunit gene in liver cells. J. Biol. Chem. 275:3841-3847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
