Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana
- PMID: 12748379
- PMCID: PMC164535
- DOI: 10.1073/pnas.1132018100
Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana
Abstract
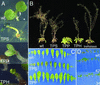
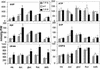
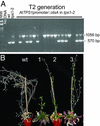
Genes for trehalose metabolism are widespread in higher plants. Insight into the physiological role of the trehalose pathway outside of resurrection plant species is lacking. To address this lack of insight, we express Escherichia coli genes for trehalose metabolism in Arabidopsis thaliana, which manipulates trehalose 6-phosphate (T6P) contents in the transgenic plants. Plants expressing otsA [encoding trehalose phosphate synthase (TPS)] accumulate T6P whereas those expressing either otsB [encoding trehalose phosphate phosphatase (TPP)] or treC [encoding trehalose phosphate hydrolase (TPH)] contain low levels of T6P. Expression of treF (encoding trehalase) yields plants with unaltered T6P content and a phenotype not distinguishable from wild type when grown on soil. The marked phenotype obtained of plants accumulating T6P is opposite to that of plants with low T6P levels obtained by expressing either TPP or TPH and consistent with a critical role for T6P in growth and development. Supplied sugar strongly inhibits growth of plants with reduced T6P content and leads to accumulation of respiratory intermediates. Remarkably, sugar improves growth of TPS expressors over wild type, a feat not previously accomplished by manipulation of metabolism. The data indicate that the T6P intermediate of the trehalose pathway controls carbohydrate utilization and thence growth via control of glycolysis in a manner analogous to that in yeast. Furthermore, embryolethal A. thaliana tps1 mutants are rescued by expression of E. coli TPS, but not by supply of trehalose, suggesting that T6P control over primary metabolism is indispensable for development.
Figures


Similar articles
-
Trehalose 6-phosphate is required for the onset of leaf senescence associated with high carbon availability.Plant Physiol. 2012 Mar;158(3):1241-51. doi: 10.1104/pp.111.191908. Epub 2012 Jan 13. Plant Physiol. 2012. PMID: 22247267 Free PMC article.
-
Altering trehalose-6-phosphate content in transgenic potato tubers affects tuber growth and alters responsiveness to hormones during sprouting.Plant Physiol. 2011 Aug;156(4):1754-71. doi: 10.1104/pp.111.179903. Epub 2011 Jun 13. Plant Physiol. 2011. PMID: 21670224 Free PMC article.
-
Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase.Proc Natl Acad Sci U S A. 2005 Aug 2;102(31):11118-23. doi: 10.1073/pnas.0503410102. Epub 2005 Jul 26. Proc Natl Acad Sci U S A. 2005. PMID: 16046541 Free PMC article.
-
Metabolism control over growth: a case for trehalose-6-phosphate in plants.J Exp Bot. 2012 May;63(9):3379-90. doi: 10.1093/jxb/err311. Epub 2011 Nov 4. J Exp Bot. 2012. PMID: 22058405 Review.
-
Is trehalose-6-phosphate a regulator of sugar metabolism in plants?J Exp Bot. 2003 Jan;54(382):533-7. doi: 10.1093/jxb/erg039. J Exp Bot. 2003. PMID: 12508064 Review.
Cited by
-
A Tale of Two Sugars: Trehalose 6-Phosphate and Sucrose.Plant Physiol. 2016 Sep;172(1):7-27. doi: 10.1104/pp.16.00417. Epub 2016 Aug 1. Plant Physiol. 2016. PMID: 27482078 Free PMC article. Review.
-
Concentration-dependent dual effects of exogenous sucrose on nitrogen metabolism in Andrographis paniculata.Sci Rep. 2022 Mar 22;12(1):4906. doi: 10.1038/s41598-022-08971-x. Sci Rep. 2022. PMID: 35318399 Free PMC article.
-
Comparative metabolic profiling of Haberlea rhodopensis, Thellungiella halophyla, and Arabidopsis thaliana exposed to low temperature.Front Plant Sci. 2013 Dec 11;4:499. doi: 10.3389/fpls.2013.00499. eCollection 2013. Front Plant Sci. 2013. PMID: 24376451 Free PMC article.
-
Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes.Plant Physiol. 2008 Apr;146(4):1834-61. doi: 10.1104/pp.107.115592. Epub 2008 Feb 27. Plant Physiol. 2008. PMID: 18305208 Free PMC article.
-
Carbohydrates in plant immunity and plant protection: roles and potential application as foliar sprays.Front Plant Sci. 2014 Nov 4;5:592. doi: 10.3389/fpls.2014.00592. eCollection 2014. Front Plant Sci. 2014. PMID: 25408694 Free PMC article. Review.
References
-
- Goddijn, O. J. & van Dun, K. (1999) Trends Plant Sci. 4, 315-319. - PubMed
-
- Bianchi, G., Gamba, A., Limiroli, R., Pozzi, N., Elster, R., Salamini, F. & Bartels, D. (1993) Physiol. Plant 87, 223-226.
-
- Drennan, P. M., Smith, M. T., Goldsworthy, D. & van Staden, J. (1993) J. Plant Physiol. 142, 493-496.
-
- Blazquez, M., Santos, E., Flores, C., Martinez-Zapater, J., Salinas, J. & Gancedo, C. (1998) Plant J. 13, 685-689. - PubMed
-
- Vogel, G., Aeschbacher, R. A., Muller, J., Boller, T. & Wiemken, A. (1998) Plant J. 13, 673-683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

