Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection
- PMID: 12748380
- PMCID: PMC164519
- DOI: 10.1073/pnas.1131929100
Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection
Abstract
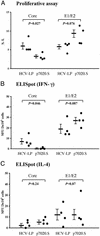
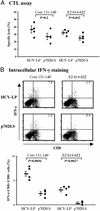
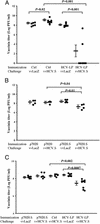
We have recently demonstrated that immunization with hepatitis C virus-like particles (HCV-LPs) generated in insect cells can elicit both humoral and cellular immune responses in BALB/c mice. Here, we evaluate the immunogenicity of HCV-LPs in HLA2.1 transgenic (AAD) mice in comparison to DNA immunization. HCV-LP immunization elicited a significantly stronger humoral immune response than DNA immunization. HCV-LP-immunized mice also developed stronger HCV-specific cellular immune responses than DNA-immunized mice as determined by using quantitative enzyme-linked immunospot (ELISpot) assay and intracellular cytokine staining. In BALB/c mice, immunization with HCV-LPs resulted in a >5 log10 reduction in vaccinia titer when challenged with a recombinant vaccinia expressing the HCV structural proteins (vvHCV.S), as compared to 1 log10 decrease in DNA immunization. In HLA2.1 transgenic mice, a 1-2 log10 reduction resulted from HCV-LP immunization, whereas no reduction was seen from DNA immunization. Adoptive transfer of lymphocytes from HCV-LP-immunized mice to naive mice provided protection against vvHCV.S challenge, and this transferred immunity can be abrogated by either CD4 or CD8 depletion. Our results suggest that HCV-LPs can induce humoral and cellular immune responses that are protective in a surrogate HCV challenge model and that a strong cellular immunity provided by both CD4 and CD8 effector lymphocytes may be important for protection from HCV infection.
Figures



Similar articles
-
Immunization with a recombinant fowlpox virus expressing a hepatitis C virus core-E1 polyprotein variant, protects mice and African green monkeys (Chlorocebus aethiops sabaeus) against challenge with a surrogate vaccinia virus.Biotechnol Appl Biochem. 2008 Oct;51(Pt 2):97-105. doi: 10.1042/BA20070182. Biotechnol Appl Biochem. 2008. PMID: 18215116
-
A novel hepatitis C virus vaccine approach using recombinant Bacillus Calmette-Guerin expressing multi-epitope antigen.Arch Virol. 2008;153(6):1021-9. doi: 10.1007/s00705-008-0082-1. Epub 2008 Apr 18. Arch Virol. 2008. PMID: 18421415
-
Genetic immunization of wild-type and hepatitis C virus transgenic mice reveals a hierarchy of cellular immune response and tolerance induction against hepatitis C virus structural proteins.J Virol. 2001 Dec;75(24):12121-7. doi: 10.1128/JVI.75.24.12121-12127.2001. J Virol. 2001. PMID: 11711603 Free PMC article.
-
Hepatitis B virus precore protein augments genetic immunizations of the truncated hepatitis C virus core in BALB/c mice.Hepatology. 2008 Jan;47(1):25-34. doi: 10.1002/hep.21992. Hepatology. 2008. PMID: 18074356
-
Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates.J Virol. 2004 Jul;78(13):6995-7003. doi: 10.1128/JVI.78.13.6995-7003.2004. J Virol. 2004. PMID: 15194776 Free PMC article.
Cited by
-
Hepatitis C genotype 1 mosaic vaccines are immunogenic in mice and induce stronger T-cell responses than natural strains.Clin Vaccine Immunol. 2013 Feb;20(2):302-5. doi: 10.1128/CVI.00605-12. Epub 2012 Dec 5. Clin Vaccine Immunol. 2013. PMID: 23221002 Free PMC article.
-
Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus.J Virol. 2004 Apr;78(8):3965-76. doi: 10.1128/jvi.78.8.3965-3976.2004. J Virol. 2004. PMID: 15047812 Free PMC article.
-
Vaccines to prevent chronic hepatitis C virus infection: current experimental and preclinical developments.J Gastroenterol. 2007 Jun;42(6):424-32. doi: 10.1007/s00535-007-2057-5. Epub 2007 Jun 29. J Gastroenterol. 2007. PMID: 17671756 Review. No abstract available.
-
Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: immunological properties.BMC Microbiol. 2014 Dec 18;14:233. doi: 10.1186/s12866-014-0233-3. BMC Microbiol. 2014. PMID: 25520151 Free PMC article.
-
Preclinical Development and Production of Virus-Like Particles As Vaccine Candidates for Hepatitis C.Front Microbiol. 2017 Dec 5;8:2413. doi: 10.3389/fmicb.2017.02413. eCollection 2017. Front Microbiol. 2017. PMID: 29259601 Free PMC article. Review.
References
-
- Liang, T. J., Rehermann, B., Seeff, L. B. & Hoofnagle, J. H. (2000) Ann. Intern. Med. 132, 296-305. - PubMed
-
- Manns, M. P., McHutchison, J. G., Gordon, S. C., Rustgi, V. K., Shiffman, M., Reindollar, R., Goodman, Z. D., Koury, K., Ling, M. & Albrecht, J. K. (2001) Lancet 358, 958-965. - PubMed
-
- Fried, M. W., Shiffman, M. L., Reddy, K. R., Smith, C., Marinos, G., Goncales, F. L., Jr., Haussinger, D., Diago, M., Carosi, G., Dhumeaux, D., et al. (2002) N. Engl. J. Med. 347, 975-982. - PubMed
-
- Baumert, T. F., Wellnitz, S., Aono, S., Satoi, J., Herion, D., Tilman Gerlach, J., Pape, G. R., Lau, J. Y., Hoofnagle, J. H., Blum, H. E. & Liang, T. J. (2000) Hepatology 32, 610-617. - PubMed
-
- Kobayashi, M., Tanaka, E., Matsumoto, A., Ichijo, T. & Kiyosawa, K. (1997) J. Gastroenterol. Hepatol. 12, 73-76. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

