Human cathepsin S, but not cathepsin L, degrades efficiently MHC class II-associated invariant chain in nonprofessional APCs
- PMID: 12748383
- PMCID: PMC164504
- DOI: 10.1073/pnas.1131604100
Human cathepsin S, but not cathepsin L, degrades efficiently MHC class II-associated invariant chain in nonprofessional APCs
Abstract
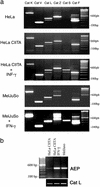
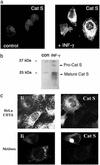
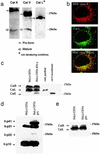
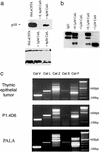
MHC class II-restricted antigen presentation plays a central role in the immune response against exogenous antigens. The association of invariant (Ii) chain with MHC class II dimers is required for proper antigen presentation to CD4+ T cells by antigen-presenting cells. MHC class II complexes first traffic through the endocytic pathway to allow Ii chain degradation and antigenic peptide loading before their arrival at the cell surface. In recent years, a considerable effort has been directed toward the identification of proteases responsible for Ii chain degradation. Targeted gene deletion in mice has allowed a precise description of the cysteine proteases involved in the last step of Ii chain degradation. By using nonspecialized cellular models expressing MHC II molecules, we are now exploring the contribution of known cysteine proteases to human Ii chain processing. Surprisingly and contrary to the situation in mouse, cathepsin S was found to be the only human cysteine protease able to efficiently degrade the Ii-p10 fragment in epithelial cells. This selectivity has implications for thymic selection and indicates that differences between man and mice are probably more profound at this level than expected.
Figures

Similar articles
-
Asparagine endopeptidase is not essential for class II MHC antigen presentation but is required for processing of cathepsin L in mice.J Immunol. 2005 Jun 1;174(11):7066-74. doi: 10.4049/jimmunol.174.11.7066. J Immunol. 2005. PMID: 15905550
-
Cathepsin S controls MHC class II-mediated antigen presentation by epithelial cells in vivo.J Immunol. 2005 Feb 1;174(3):1205-12. doi: 10.4049/jimmunol.174.3.1205. J Immunol. 2005. PMID: 15661874
-
Role for cathepsin F in invariant chain processing and major histocompatibility complex class II peptide loading by macrophages.J Exp Med. 2000 Apr 3;191(7):1177-86. doi: 10.1084/jem.191.7.1177. J Exp Med. 2000. PMID: 10748235 Free PMC article.
-
Lysosomal cysteine proteases and antigen presentation.Ernst Schering Res Found Workshop. 2006;(56):81-95. doi: 10.1007/3-540-37673-9_5. Ernst Schering Res Found Workshop. 2006. PMID: 16329647 Review.
-
Proteases involved in MHC class II antigen presentation.Immunol Rev. 1999 Dec;172:109-20. doi: 10.1111/j.1600-065x.1999.tb01360.x. Immunol Rev. 1999. PMID: 10631941 Review.
Cited by
-
Expression and characterization of cathepsin P.Biochem J. 2004 Mar 1;378(Pt 2):657-63. doi: 10.1042/BJ20031548. Biochem J. 2004. PMID: 14629193 Free PMC article.
-
Cathepsin S Is More Abundant in Serum of Mycobacterium avium subsp. paratuberculosis-Infected Dairy Cows.Metabolites. 2024 Apr 11;14(4):215. doi: 10.3390/metabo14040215. Metabolites. 2024. PMID: 38668343 Free PMC article.
-
Cathepsin L promotes secretory IgA response by participating in antigen presentation pathways during Mycoplasma Hyopneumoniae infection.PLoS One. 2019 Apr 15;14(4):e0215408. doi: 10.1371/journal.pone.0215408. eCollection 2019. PLoS One. 2019. PMID: 30986254 Free PMC article.
-
Differential Role of Cathepsins S and B In Hepatic APC-Mediated NKT Cell Activation and Cytokine Secretion.Front Immunol. 2018 Feb 28;9:391. doi: 10.3389/fimmu.2018.00391. eCollection 2018. Front Immunol. 2018. PMID: 29541077 Free PMC article.
-
A soluble form of the high affinity IgE receptor, Fc-epsilon-RI, circulates in human serum.PLoS One. 2011 Apr 22;6(4):e19098. doi: 10.1371/journal.pone.0019098. PLoS One. 2011. PMID: 21544204 Free PMC article.
References
-
- Hiltbold, E. M & Roche, P. A. (2002) Curr. Opin. Immunol. 14, 30–35. - PubMed
-
- Neefjes, J. J., Stollorz V., Peters P. J., Geuze H. J. & Ploegh, H. L. (1990) Cell 61, 171–183. - PubMed
-
- Stumptner-Cuvelette, P. & Benaroch, P. (2002) Biochim. Biophys. Acta 1542, 1–13. - PubMed
-
- Kropshofer, H., Arndt, S. O., Moldenhauer, G., Hammerling, G. J. & Vogt. A. B. (1997) Immunity 6, 293–302. - PubMed
-
- Hsieh, C. S., deRoos, P., Honey, K., Beers, C. & Rudensky, A. Y. (2002) J. Immunol. 168, 2618–2625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

