Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana
- PMID: 12748386
- PMCID: PMC164538
- DOI: 10.1073/pnas.1031755100
Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana
Abstract
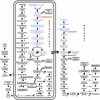
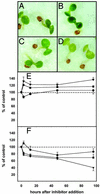
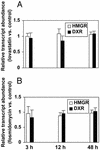
In plants, the formation of isopentenyl diphosphate and dimethylallyl diphosphate, the central intermediates in the biosynthesis of isoprenoids, is compartmentalized: the mevalonate (MVA) pathway, which is localized to the cytosol, is responsible for the synthesis of sterols, certain sesquiterpenes, and the side chain of ubiquinone; in contrast, the recently discovered MVA-independent pathway, which operates in plastids, is involved in providing the precursors for monoterpenes, certain sesquiterpenes, diterpenes, carotenoids, and the side chains of chlorophylls and plastoquinone. Specific inhibitors of the MVA pathway (lovastatin) and the MVA-independent pathway (fosmidomycin) were used to perturb biosynthetic flux in Arabidopsis thaliana seedlings. The interaction between both pathways was studied at the transcriptional level by using GeneChip (Affymetrix) microarrays and at the metabolite level by assaying chlorophylls, carotenoids, and sterols. Treatment of seedlings with lovastatin resulted in a transient decrease in sterol levels and a transient increase in carotenoid as well as chlorophyll levels. After the initial drop, sterol amounts in lovastatin-treated seedlings recovered to levels above controls. As a response to fosmidomycin treatment, a transient increase in sterol levels was observed, whereas chlorophyll and carotenoid amounts decreased dramatically when compared with controls. At 96 h after fosmidomycin addition, the levels of all metabolites assayed (sterols, chlorophylls, and carotenoids) were substantially lower than in controls. Interestingly, these inhibitor-mediated changes were not reflected in altered gene expression levels of the genes involved in sterol, chlorophyll, and carotenoid metabolism. The lack of correlation between gene expression patterns and the accumulation of isoprenoid metabolites indicates that posttranscriptional processes may play an important role in regulating flux through isoprenoid metabolic pathways.
Figures
References
-
- Harborne, J. B. (1991) in Ecological Chemistry and Biochemistry of Plant Terpenoids, eds. Harborne, J. B. & Tomas-Barberan, R. A. (Clarendon, Oxford), pp. 399-426.
-
- Lichtenthaler, H. K., Schwender, J., Disch, A. & Rohmer, M. (1997) FEBS Lett. 400, 271-274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

