Dictyostelium and Acanthamoeba myosin II assembly domains go to the cleavage furrow of Dictyostelium myosin II-null cells
- PMID: 12748387
- PMCID: PMC164475
- DOI: 10.1073/pnas.0732155100
Dictyostelium and Acanthamoeba myosin II assembly domains go to the cleavage furrow of Dictyostelium myosin II-null cells
Abstract
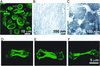
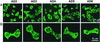
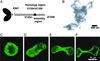
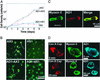
How myosin II localizes to the cleavage furrow of dividing cells is largely unknown. We show here that a 283-residue protein, assembly domain (AD)1, corresponding to the AD in the tail of Dictyostelium myosin II assembles into bundles of long tubules when expressed in myosin II-null cells and localizes to the cleavage furrow of dividing cells. AD1 mutants that do not polymerize in vitro do not go to the cleavage furrow in vivo. An assembly-competent polypeptide corresponding to the C-terminal 256 residues of Acanthamoeba myosin II also goes to the cleavage furrow of Dictyostelium myosin II-null cells. When overexpressed in wild-type cells, AD1 colocalizes with endogenous myosin II (possibly as a copolymer) in interphase, motile, and dividing cells and under caps of Con A receptors but has no effect on myosin II-dependent functions. These results suggest that neither a specific sequence, other than that required for polymerization, nor interaction with other proteins is required for localization of myosin II to the cleavage furrow.
Figures




Similar articles
-
Tail chimeras of Dictyostelium myosin II support cytokinesis and other myosin II activities but not full development.J Cell Sci. 2002 Nov 15;115(Pt 22):4237-49. doi: 10.1242/jcs.00112. J Cell Sci. 2002. PMID: 12376556
-
Dictyostelium myosin bipolar thick filament formation: importance of charge and specific domains of the myosin rod.PLoS Biol. 2004 Nov;2(11):e356. doi: 10.1371/journal.pbio.0020356. Epub 2004 Oct 19. PLoS Biol. 2004. PMID: 15492777 Free PMC article.
-
Genetic suppression of a phosphomimic myosin II identifies system-level factors that promote myosin II cleavage furrow accumulation.Mol Biol Cell. 2014 Dec 15;25(25):4150-65. doi: 10.1091/mbc.E14-08-1322. Epub 2014 Oct 15. Mol Biol Cell. 2014. PMID: 25318674 Free PMC article.
-
On the mechanism of cleavage furrow ingression in Dictyostelium.Cell Struct Funct. 2001 Dec;26(6):577-84. doi: 10.1247/csf.26.577. Cell Struct Funct. 2001. PMID: 11942612 Review.
-
Signaling pathways regulating Dictyostelium myosin II.J Muscle Res Cell Motil. 2002;23(7-8):703-18. doi: 10.1023/a:1024467426244. J Muscle Res Cell Motil. 2002. PMID: 12952069 Review.
Cited by
-
Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium.Proc Natl Acad Sci U S A. 2005 Feb 1;102(5):1472-7. doi: 10.1073/pnas.0409528102. Epub 2005 Jan 25. Proc Natl Acad Sci U S A. 2005. PMID: 15671182 Free PMC article.
-
Cytokinesis depends on the motor domains of myosin-II in fission yeast but not in budding yeast.Mol Biol Cell. 2005 Nov;16(11):5346-55. doi: 10.1091/mbc.e05-07-0601. Epub 2005 Sep 7. Mol Biol Cell. 2005. PMID: 16148042 Free PMC article.
-
14-3-3, an integrator of cell mechanics and cytokinesis.Small GTPases. 2010 Nov;1(3):165-169. doi: 10.4161/sgtp.1.3.14432. Small GTPases. 2010. PMID: 21686271 Free PMC article.
-
Phosphorylation of actin Tyr-53 inhibits filament nucleation and elongation and destabilizes filaments.Proc Natl Acad Sci U S A. 2006 Sep 12;103(37):13694-9. doi: 10.1073/pnas.0606321103. Epub 2006 Aug 30. Proc Natl Acad Sci U S A. 2006. PMID: 16945900 Free PMC article.
-
S-adenosylhomocysteine hydrolase is localized at the front of chemotaxing cells, suggesting a role for transmethylation during migration.Proc Natl Acad Sci U S A. 2006 Dec 26;103(52):19788-93. doi: 10.1073/pnas.0609385103. Epub 2006 Dec 15. Proc Natl Acad Sci U S A. 2006. PMID: 17172447 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

