The receptor kinases LePRK1 and LePRK2 associate in pollen and when expressed in yeast, but dissociate in the presence of style extract
- PMID: 12748390
- PMCID: PMC164537
- DOI: 10.1073/pnas.0631728100
The receptor kinases LePRK1 and LePRK2 associate in pollen and when expressed in yeast, but dissociate in the presence of style extract
Abstract
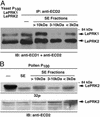
After pollen grains germinate on the stigma, pollen tubes traverse the extracellular matrix of the style on their way to the ovules. We previously characterized two pollen-specific, receptor-like kinases, LePRK1 and LePRK2, from tomato (Lycopersicon esculentum). Their structure and immunolocalization pattern and the specific dephosphorylation of LePRK2 suggested that these kinases might interact with signaling molecules in the style extracellular matrix. Here, we show that LePRK1 and LePRK2 can be coimmunoprecipitated from pollen or when expressed together in yeast. In yeast, their association requires LePRK2 kinase activity. In pollen, LePRK1 and LePRK2 are found in an approximately 400-kDa protein complex that persists on pollen germination, but this complex is disrupted when pollen is germinated in vitro in the presence of style extract. In yeast, the addition of style extract also disrupts the interaction between LePRK1 and LePRK2. Fractionation of the style extract reveals that the disruption activity is enriched in the 3- to 10-kDa fraction. A component(s) in this fraction also is responsible for the specific dephosphorylation of LePRK2. The style component(s) that dephosphorylates LePRK2 is likely to be a heat-stable peptide that is present in exudate from the style. The generally accepted model of receptor kinase signaling involves binding of a ligand to extracellular domains of receptor kinases and subsequent activation of the signaling pathway by receptor autophosphorylation. In contrast to this typical scenario, we propose that a putative style ligand transduces the signal in pollen tubes by triggering the specific dephosphorylation of LePRK2, followed by dissociation of the LePRK complex.
Figures





Similar articles
-
Pollen tube localization implies a role in pollen-pistil interactions for the tomato receptor-like protein kinases LePRK1 and LePRK2.Plant Cell. 1998 Mar;10(3):319-30. doi: 10.1105/tpc.10.3.319. Plant Cell. 1998. PMID: 9501107 Free PMC article.
-
Oligomerization studies show that the kinase domain of the tomato pollen receptor kinase LePRK2 is necessary for interaction with LePRK1.Plant Physiol Biochem. 2012 Apr;53:40-5. doi: 10.1016/j.plaphy.2012.01.008. Epub 2012 Jan 14. Plant Physiol Biochem. 2012. PMID: 22306355
-
A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2.Plant Cell. 2002 Sep;14(9):2277-87. doi: 10.1105/tpc.003103. Plant Cell. 2002. PMID: 12215520 Free PMC article.
-
MAP kinases in pollen.Results Probl Cell Differ. 2000;27:39-51. doi: 10.1007/978-3-540-49166-8_4. Results Probl Cell Differ. 2000. PMID: 10533197 Review.
-
The mechanisms of pollination and fertilization in plants.Annu Rev Cell Dev Biol. 2002;18:81-105. doi: 10.1146/annurev.cellbio.18.012502.083438. Epub 2002 Apr 2. Annu Rev Cell Dev Biol. 2002. PMID: 12142268 Review.
Cited by
-
Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth.Plant Cell. 2006 Feb;18(2):366-81. doi: 10.1105/tpc.105.036434. Epub 2006 Jan 13. Plant Cell. 2006. PMID: 16415208 Free PMC article.
-
STIL, a peculiar molecule from styles, specifically dephosphorylates the pollen receptor kinase LePRK2 and stimulates pollen tube growth in vitro.BMC Plant Biol. 2010 Feb 22;10:33. doi: 10.1186/1471-2229-10-33. BMC Plant Biol. 2010. PMID: 20175921 Free PMC article.
-
Tomato Pistil Factor STIG1 Promotes in Vivo Pollen Tube Growth by Binding to Phosphatidylinositol 3-Phosphate and the Extracellular Domain of the Pollen Receptor Kinase LePRK2.Plant Cell. 2014 Jun;26(6):2505-2523. doi: 10.1105/tpc.114.123281. Epub 2014 Jun 17. Plant Cell. 2014. PMID: 24938288 Free PMC article.
-
Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis.Nature. 2016 Mar 10;531(7593):245-8. doi: 10.1038/nature17413. Nature. 2016. PMID: 26961657
-
Kinase Partner Protein Plays a Key Role in Controlling the Speed and Shape of Pollen Tube Growth in Tomato.Plant Physiol. 2020 Dec;184(4):1853-1869. doi: 10.1104/pp.20.01081. Epub 2020 Oct 5. Plant Physiol. 2020. PMID: 33020251 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

