Growth-promoting effect of muscarinic acetylcholine receptors in colon cancer cells
- PMID: 12748850
- PMCID: PMC12161958
- DOI: 10.1007/s00432-003-0433-y
Growth-promoting effect of muscarinic acetylcholine receptors in colon cancer cells
Abstract
Purpose: G-protein-coupled receptors are known to mediate cell growth via divergent signaling pathways. It has been reported that colon cancer cells express muscarinic acetylcholine receptor (mAChR) although their functional role is largely unknown. The aim of this study is to elucidate possible mechanisms responsible for the growth-promoting effect of mAChRs in colon cancer cells by using colon cancer cell line T84.
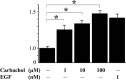
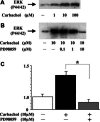
Methods: Carbachol, a stable mAChR agonist, dose-dependently induced cell growth with a maximal effect observed at 100 microM, equipotent with 1 nM EGF. 4-DAMP, a specific antagonist of subtype 3 mAChR, inhibited the stimulatory effect by carbachol, suggesting that the growth-promoting effect was receptor-mediated. Carbachol also dose-dependently stimulated extracellular signal-regulated protein kinase (ERK) activation. This effect was inhibited by PD98059, an inhibitor of extracellular signal-regulated protein kinase kinase, which also blocked carbachol activation of cell proliferation, indicating that the p21Ras-ERK pathway is an important signaling cascade in the mitogenic effect. To investigate how mAChR activated the p21Ras-ERK pathway, transactivation of epidermal growth factor receptor (EGFR) was examined.
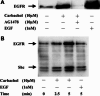
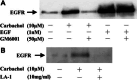
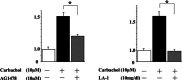
Results: Carbachol induced tyrosine phosphorylation of EGFR, which was abolished by an EGFR tyrosine kinase inhibitor AG1478. Transactivation by carbachol was also abrogated by a metalloproteinases (MMPs) inhibitor GM6001 or an EGFR-blocking antibody (LA-1), suggesting that binding of EGFR ligand(s) produced by MMPs may initiate transactivation in a manner dependent on EGFR tyrosine kinase. The tyrosine-phosphorylated EGFR was immunoprecipitated together with GRB2 and tyrosine-phosphorylated Shc, indicating that transactivated EGFR is able to generate downstream signals. AG 1478 and LA-1 inhibited carbachol stimulation of cell growth.
Conclusions: Taken together, our results indicate that the growth-promoting effect of subtype 3 mAChR in colon cancer cells may depend on transactivated EGFR-ERK pathways. EGFR not only receives external stimuli but also serves as a scaffold for downstream signaling molecules.
Figures
Similar articles
-
Muscarinic acetylcholine receptor-mediated phosphorylation of extracellular signal-regulated kinase in HSY salivary ductal cells involves distinct signaling pathways.J Oral Biosci. 2024 Jun;66(2):447-455. doi: 10.1016/j.job.2024.02.002. Epub 2024 Feb 8. J Oral Biosci. 2024. PMID: 38336259
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T84 cells. Implications for carbachol-stimulated chloride secretion.J Biol Chem. 1998 Oct 16;273(42):27111-7. doi: 10.1074/jbc.273.42.27111. J Biol Chem. 1998. PMID: 9765228
-
The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors.J Cancer Res Clin Oncol. 2005 Oct;131(10):639-48. doi: 10.1007/s00432-005-0002-7. Epub 2005 Oct 20. J Cancer Res Clin Oncol. 2005. PMID: 16091975 Free PMC article.
-
Interventions for the treatment of oral and oropharyngeal cancers: targeted therapy and immunotherapy.Cochrane Database Syst Rev. 2015 Dec 1;2015(12):CD010341. doi: 10.1002/14651858.CD010341.pub2. Cochrane Database Syst Rev. 2015. PMID: 26625332 Free PMC article.
Cited by
-
Tumor progression: the neuronal input.Ann Transl Med. 2018 Mar;6(5):89. doi: 10.21037/atm.2018.01.01. Ann Transl Med. 2018. PMID: 29666812 Free PMC article. Review.
-
Muscarinic receptor agonists and antagonists: effects on cancer.Handb Exp Pharmacol. 2012;(208):451-68. doi: 10.1007/978-3-642-23274-9_19. Handb Exp Pharmacol. 2012. PMID: 22222710 Free PMC article. Review.
-
Cholinergic Mechanisms in Gastrointestinal Neoplasia.Int J Mol Sci. 2024 May 13;25(10):5316. doi: 10.3390/ijms25105316. Int J Mol Sci. 2024. PMID: 38791353 Free PMC article. Review.
-
EGFR and PKC are involved in the activation of ERK1/2 and p90 RSK and the subsequent proliferation of SNU-407 colon cancer cells by muscarinic acetylcholine receptors.Mol Cell Biochem. 2012 Nov;370(1-2):191-8. doi: 10.1007/s11010-012-1410-z. Epub 2012 Aug 3. Mol Cell Biochem. 2012. PMID: 22865467
-
Comparative proteomic analysis implicates eEF2 as a novel target of PI3Kγ in the MDA-MB-231 metastatic breast cancer cell line.Proteome Sci. 2013 Jan 15;11(1):4. doi: 10.1186/1477-5956-11-4. Proteome Sci. 2013. PMID: 23320409 Free PMC article.
References
-
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E (1997) Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347–350 - PubMed
-
- Ashkenazi A, Ramachandran J, Capon DJ (1989) Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature 340:146–150 - PubMed
-
- Binder HJ, Sandle GI (1987) Electrolyte absorption and secretion in the mammalian colon. In: Johnson LR (ed) Physiology of the gastrointestinal tract. Raven, New York, pp 1389–1418
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

