Spherical aggregates of beta-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3beta
- PMID: 12750461
- PMCID: PMC164453
- DOI: 10.1073/pnas.1237107100
Spherical aggregates of beta-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3beta
Abstract
beta-Amyloid (Abeta) acquires toxicity by self-aggregation. To identify and characterize the toxic form(s) of Abeta aggregates, we examined in vitro aggregation conditions by using large quantities of homogenous, chemically synthesized Abeta1-40 peptide. We found that slow rotation of Abeta1-40 solution reproducibly gave self-aggregated Abeta1-40 containing a stable and highly toxic moiety. Examination of the aggregates purified by glycerol-gradient centrifugation by atomic force microscopy and transmission electron microscopy revealed that the toxic moiety is a perfect sphere, which we call amylospheroid (ASPD). Other Abeta1-40 aggregates, including fibrils, were nontoxic. Correlation studies between toxicity and sphere size indicate that 10- to 15-nm ASPD was highly toxic, whereas ASPD <10 nm was nontoxic. A positive correlation between the toxicity and ASPD >10 nm also appeared to exist when Abeta1-42 formed ASPD by slow rotation. However, Abeta1-42-ASPD formed more rapidly, killed neurons at lower concentrations, and showed approximately 100-fold-higher toxicity than Abeta1-40-ASPD. The toxic ASPD was associated with SDS-resistant oligomeric bands in immunoblotting, which were absent in nontoxic ASPD. Because the formation of ASPD was not disturbed by pentapeptides that break beta-sheet interactions, Abeta may form ASPD through a pathway that is at least partly distinct from that of fibril formation. Inhibition experiments with lithium suggest the involvement of tau protein kinase I/glycogen synthase kinase-3beta in the early stages of ASPD-induced neurodegeneration. Here we describe the identification and characterization of ASPD and discuss its possible role in the neurodegeneration in Alzheimer's disease.
Figures

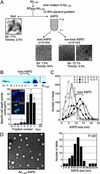
 ). Hereafter, we designated toxic Aβ1–40 aggregates formed by slowly rotating Aβ1–40 solution (350 μMin50% PBS) as SR350–Aβ1–40. (B) The structure and toxicity of Aβ1–40 aggregates formed by slow rotation. At the indicated time, aliquots were removed from SR350–Aβ1–40 for transmission electron microscopy and MTT assay (n = 9) (5 μM). (C) Aβ1–40 solutions [350 μM (SR350–Aβ1–40), 50 μM (SR50–Aβ1–40), and 1 μM (SR1–Aβ1–40)] each were rotated slowly for 7 days, and the toxicity was assessed (n = 7). Thioflavine T assay (20 μM; Sigma; excitation at 445 nm and emission at 485 nm; ref. 48) determined fibril content in freshly dissolved Aβ1–40 and in each SR–Aβ1–40 (n = 6). (D) SR350–Aβ1–40 was prepared with or without KLVFF or LPFFD. The toxicity was determined by MTT assay (n = 9) or propidium iodide staining (Right). Image of SR350–Aβ1–40 with LPFFD is shown (bar, 100 nm). Also shown are SR350–Aβ1–40 (○) with KLVFF (•) and with LPFFD (
). Hereafter, we designated toxic Aβ1–40 aggregates formed by slowly rotating Aβ1–40 solution (350 μMin50% PBS) as SR350–Aβ1–40. (B) The structure and toxicity of Aβ1–40 aggregates formed by slow rotation. At the indicated time, aliquots were removed from SR350–Aβ1–40 for transmission electron microscopy and MTT assay (n = 9) (5 μM). (C) Aβ1–40 solutions [350 μM (SR350–Aβ1–40), 50 μM (SR50–Aβ1–40), and 1 μM (SR1–Aβ1–40)] each were rotated slowly for 7 days, and the toxicity was assessed (n = 7). Thioflavine T assay (20 μM; Sigma; excitation at 445 nm and emission at 485 nm; ref. 48) determined fibril content in freshly dissolved Aβ1–40 and in each SR–Aβ1–40 (n = 6). (D) SR350–Aβ1–40 was prepared with or without KLVFF or LPFFD. The toxicity was determined by MTT assay (n = 9) or propidium iodide staining (Right). Image of SR350–Aβ1–40 with LPFFD is shown (bar, 100 nm). Also shown are SR350–Aβ1–40 (○) with KLVFF (•) and with LPFFD ( ). Data represent the mean ± SE. *, Significant difference from the control. The results suggest that toxicity of SR350–Aβ1–40 is associated with the spherical structures (B, 4 h), which we call ASPD.
). Data represent the mean ± SE. *, Significant difference from the control. The results suggest that toxicity of SR350–Aβ1–40 is associated with the spherical structures (B, 4 h), which we call ASPD.

References
-
- Glenner, G. G. & Wong, C. W. (1984) Biochem. Biophys. Res. Commun. 120, 885–890. - PubMed
-
- Selkoe, D. J. (2001) Physiol. Rev. 81, 741–766. - PubMed
-
- Schenk, D., Barbour, R., Dunn, W., Gordon, G., Grajeda, H., Guido, T., Hu, K., Huang, J., Johnson-Wood, K., Khan, K., et al. (1999) Nature 400, 173–177. - PubMed
-
- Janus, C., Pearson, J., McLaurin, J., Mathews, P. M., Jiang, Y., Schmidt, S. D., Chishti, M. A., Horne, P., Heslin, D., French, J., et al. (2000) Nature 408, 979–982. - PubMed
-
- Morgan, D., Diamond, D. M., Gottschall, P. E., Ugen, K. E., Dickey, C., Hardy, J., Duff, K., Jantzen, P., DiCarlo, G., Wilcock, D., et al. (2000) Nature 408, 982–985. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

