Inactivation of Mg chelatase during transition from anaerobic to aerobic growth in Rhodobacter capsulatus
- PMID: 12754222
- PMCID: PMC155376
- DOI: 10.1128/JB.185.11.3249-3258.2003
Inactivation of Mg chelatase during transition from anaerobic to aerobic growth in Rhodobacter capsulatus
Abstract
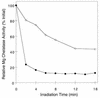
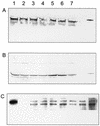
The facultative photosynthetic bacterium Rhodobacter capsulatus can adapt from an anaerobic photosynthetic mode of growth to aerobic heterotrophic metabolism. As this adaptation occurs, the cells must rapidly halt bacteriochlorophyll synthesis to prevent phototoxic tetrapyrroles from accumulating, while still allowing heme synthesis to continue. A likely control point is Mg chelatase, the enzyme that diverts protoporphyrin IX from heme biosynthesis toward the bacteriochlorophyll biosynthetic pathway by inserting Mg(2+) to form Mg-protoporphyrin IX. Mg chelatase is composed of three subunits that are encoded by the bchI, bchD, and bchH genes in R. capsulatus. We report that BchH is the rate-limiting component of Mg chelatase activity in cell extracts. BchH binds protoporphyrin IX, and BchH that has been expressed and purified from Escherichia coli is red in color due to the bound protoporphyrin IX. Recombinant BchH is rapidly inactivated by light in the presence of O(2), and the inactivation results in the formation of a covalent adduct between the protein and the bound protoporphyrin IX. When photosynthetically growing R. capsulatus cells are transferred to aerobic conditions, Mg chelatase is rapidly inactivated, and BchH is the component that is most rapidly inactivated in vivo when cells are exposed to aerobic conditions. The light- and O(2)-stimulated inactivation of BchH could account for the rapid inactivation of Mg chelatase in vivo and provide a mechanism for inhibiting the synthesis of bacteriochlorophyll during adaptation of photosynthetically grown cells to aerobic conditions while still allowing heme synthesis to occur for aerobic respiration.
Figures


Similar articles
-
Heterologous expression of the Rhodobacter capsulatus BchI, -D, and -H genes that encode magnesium chelatase subunits and characterization of the reconstituted enzyme.J Biol Chem. 1998 Dec 18;273(51):34206-13. doi: 10.1074/jbc.273.51.34206. J Biol Chem. 1998. PMID: 9852082
-
BchJ and BchM interact in a 1 : 1 ratio with the magnesium chelatase BchH subunit of Rhodobacter capsulatus.FEBS J. 2010 Nov;277(22):4709-21. doi: 10.1111/j.1742-4658.2010.07877.x. Epub 2010 Oct 19. FEBS J. 2010. PMID: 20955518
-
Kinetic analyses of the magnesium chelatase provide insights into the mechanism, structure, and formation of the complex.J Biol Chem. 2008 Nov 14;283(46):31294-302. doi: 10.1074/jbc.M805792200. Epub 2008 Sep 12. J Biol Chem. 2008. PMID: 18790730
-
Biosynthesis of chlorophylls from protoporphyrin IX.Nat Prod Rep. 2003 Jun;20(3):327-41. doi: 10.1039/b110549n. Nat Prod Rep. 2003. PMID: 12828371 Review.
-
The tetrapyrrole biosynthetic pathway and its regulation in Rhodobacter capsulatus.Adv Exp Med Biol. 2010;675:229-50. doi: 10.1007/978-1-4419-1528-3_13. Adv Exp Med Biol. 2010. PMID: 20532744 Free PMC article. Review.
Cited by
-
ATPase activity associated with the magnesium chelatase H-subunit of the chlorophyll biosynthetic pathway is an artefact.Biochem J. 2006 Dec 15;400(3):477-84. doi: 10.1042/BJ20061103. Biochem J. 2006. PMID: 16928192 Free PMC article.
-
GUN4-porphyrin complexes bind the ChlH/GUN5 subunit of Mg-Chelatase and promote chlorophyll biosynthesis in Arabidopsis.Plant Cell. 2011 Apr;23(4):1449-67. doi: 10.1105/tpc.110.082503. Epub 2011 Apr 5. Plant Cell. 2011. PMID: 21467578 Free PMC article.
-
GUN4 appeared early in cyanobacterial evolution.PNAS Nexus. 2023 Apr 12;2(5):pgad131. doi: 10.1093/pnasnexus/pgad131. eCollection 2023 May. PNAS Nexus. 2023. PMID: 37152672 Free PMC article.
-
Structure of the cyanobacterial Magnesium Chelatase H subunit determined by single particle reconstruction and small-angle X-ray scattering.J Biol Chem. 2012 Feb 10;287(7):4946-56. doi: 10.1074/jbc.M111.308239. Epub 2011 Dec 15. J Biol Chem. 2012. PMID: 22179610 Free PMC article.
-
Mutational analysis of three bchH paralogs in (bacterio-)chlorophyll biosynthesis in Chlorobaculum tepidum.Photosynth Res. 2009 Jul;101(1):21-34. doi: 10.1007/s11120-009-9460-0. Epub 2009 Jul 1. Photosynth Res. 2009. PMID: 19568953
References
-
- Bauer, C. E., and T. H. Bird. 1996. Regulatory circuits controlling photosynthesis gene expression. Cell 85:5-8. - PubMed
-
- Bollivar, D. W., J. Y. Suzuki, J. T. Beatty, J. M. Dobrowolski, and C. E. Bauer. 1994. Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J. Mol. Biol. 237:622-640. - PubMed
-
- Cohen-Bazire, G., W. R. Sistrom, and R. Y. Stanier. 1957. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J. Cell. Comp. Physiol. 49:25-68. - PubMed
-
- Dalziel, K. 1969. Porphyrins and related compounds, p. 128-145. In R. M. C. Dawson (ed.), Data for biochemical research. Clarendon Press, Oxford, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

