Concentration and assembly of the division ring proteins FtsZ, FtsA, and ZipA during the Escherichia coli cell cycle
- PMID: 12754232
- PMCID: PMC155373
- DOI: 10.1128/JB.185.11.3344-3351.2003
Concentration and assembly of the division ring proteins FtsZ, FtsA, and ZipA during the Escherichia coli cell cycle
Abstract
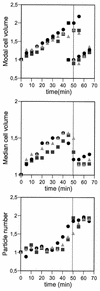
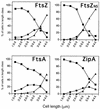
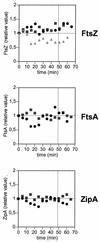
The concentration of the cell division proteins FtsZ, FtsA, and ZipA and their assembly into a division ring during the Escherichia coli B/r K cell cycle have been measured in synchronous cultures obtained by the membrane elution technique. Immunostaining of the three proteins revealed no organized structure in newly born cells. In a culture with a doubling time of 49 min, assembly of the Z ring started around minute 25 and was detected first as a two-dot structure that became a sharp band before cell constriction. FtsA and ZipA localized into a division ring following the same pattern and time course as FtsZ. The concentration (amount relative to total mass) of the three proteins remained constant during one complete cell cycle, showing that assembly of a division ring is not driven by changes in the concentration of these proteins. Maintenance of the Z ring during the process of septation is a dynamic energy-dependent event, as evidenced by its disappearance in cells treated with sodium azide.
Figures


References
-
- Bouché, F., and J. P. Bouché. 1989. Genetic evidence that DicF, a second division inhibitor encoded by the Escherichia coli dicB operon, is probably RNA. Mol. Microbiol. 3:991-994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

