Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque
- PMID: 12754239
- PMCID: PMC155391
- DOI: 10.1128/JB.185.11.3400-3409.2003
Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque
Abstract
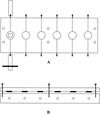
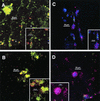
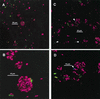
Streptococci and actinomyces that initiate colonization of the tooth surface frequently coaggregate with each other as well as with other oral bacteria. These observations have led to the hypothesis that interbacterial adhesion influences spatiotemporal development of plaque. To assess the role of such interactions in oral biofilm formation in vivo, antibodies directed against bacterial surface components that mediate coaggregation interactions were used as direct immunofluorescent probes in conjunction with laser confocal microscopy to determine the distribution and spatial arrangement of bacteria within intact human plaque formed on retrievable enamel chips. In intrageneric coaggregation, streptococci such as Streptococcus gordonii DL1 recognize receptor polysaccharides (RPS) borne on other streptococci such as Streptococcus oralis 34. To define potentially interactive subsets of streptococci in the developing plaque, an antibody against RPS (anti-RPS) was used together with an antibody against S. gordonii DL1 (anti-DL1). These antibodies reacted primarily with single cells in 4-h-old plaque and with mixed-species microcolonies in 8-h-old plaque. Anti-RPS-reactive bacteria frequently formed microcolonies with anti-DL1-reactive bacteria and with other bacteria distinguished by general nucleic acid stains. In intergeneric coaggregation between streptococci and actinomyces, type 2 fimbriae of actinomyces recognize RPS on the streptococci. Cells reactive with antibody against type 2 fimbriae of Actinomyces naeslundii T14V (anti-type-2) were much less frequent than either subset of streptococci. However, bacteria reactive with anti-type-2 were seen in intimate association with anti-RPS-reactive cells. These results are the first direct demonstration of coaggregation-mediated interactions during initial plaque accumulation in vivo. Further, these results demonstrate the spatiotemporal development and prevalence of mixed-species communities in early dental plaque.
Figures



References
-
- Buswell, C. M., Y. M. Herlihy, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1997. Coaggregation amongst aquatic biofilm bacteria. J. Appl. Microbiol. 83:477-484.
-
- Cisar, J. O., M. J. Brennan, and A. L. Sandberg. 1985. Lectin-specific interaction of Actinomyces fimbriae with oral streptococci, p. 159-163. In S. E. Mergenhagen and B. Rosan (ed.), Molecular basis of oral microbial adhesion. American Society for Microbiology, Washington, D.C.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

