Purification and characterization of a transmembrane domain-deleted form of lecithin retinol acyltransferase
- PMID: 12755610
- PMCID: PMC5507194
- DOI: 10.1021/bi0342416
Purification and characterization of a transmembrane domain-deleted form of lecithin retinol acyltransferase
Abstract
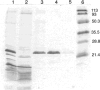
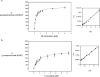
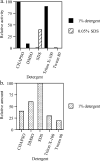
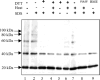
Lecithin retinol acyltransferase (LRAT) catalyzes the esterification of all-trans-retinol into all-trans-retinyl ester, an essential reaction in the vertebrate visual cycle. Since all-trans-retinyl esters are the substrates for the isomerization reaction that generates 11-cis-retinoids, this esterification reaction is essential in the operation of the visual cycle. In addition, LRAT is the founder member of a series of proteins, which are of novel sequence and have unknown functions. Native LRAT is an integral membrane protein and has never been purified. To obtain a pure LRAT, the N- and C-transmembrane termini were deleted and replaced with a poly His tag for the purpose of purification. This truncated form of LRAT, referred to as tLRAT, has been expressed in bacteria and fully purified. tLRAT is catalytically active and processes all-trans-retinol at least 10-fold more efficiently than 11-cis-retinol, the precursor to the visual chromophore. While tLRAT can be robustly expressed in bacteria, it requires detergent for extraction, as the enzyme still contains hydrophobic domains, which may interact. Indeed, tLRAT can oligomerize and forms dimers. Native LRAT also forms functional homodimers. These studies pave the way for the preparation of large-scale amounts of pure tLRAT for further mechanistic and structural studies.
Figures



References
-
- MacDonald PN, Ong DE. J. Biol. Chem. 1988;263:12478–12482. - PubMed
-
- Barry R, Cañada FJ, Rando RR. J. Biol. Chem. 1989;264:9231–9238. - PubMed
-
- Saari JC, Bredberg DL. J. Biol. Chem. 1989;264:8636–8640. - PubMed
-
- Ruiz A, Winston A, Lim Y-H, Gilbert BZ, Rando RR, Bok D. J. Biol. Chem. 1999;274:3834–3841. - PubMed
-
- MacDonald PN, Ong DE. Biochem. Biophys. Res. Commun. 1988;156:157–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

