The effects of estrogen, its antagonist ICI 182, 780, and interferon-tau on the expression of estrogen receptors and integrin alphaV beta 3 on cycle day 16 in bovine endometrium
- PMID: 12756058
- PMCID: PMC155788
- DOI: 10.1186/1477-7827-1-38
The effects of estrogen, its antagonist ICI 182, 780, and interferon-tau on the expression of estrogen receptors and integrin alphaV beta 3 on cycle day 16 in bovine endometrium
Abstract
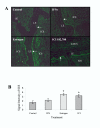
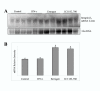
We have shown previously that downregulation of intercaruncular stromal integrin alphavbeta3 in bovine endometrium on day 16 of the estrous cycle coincided with the antibody recognition of estrogen receptors (ER) in the luminal epithelium. In pregnancy, these changes were not observed. Our hypothesis was that on day 16 of the estrous cycle, estrogen from the dominant follicle causes a reduction in integrin alphavbeta3 and affects ERalpha in the luminal epithelium. The pregnancy recognition protein, interferon-tau (IFN-tau), may prevent downregulation of integrin alphavbeta3 and suppress ERalpha expression in the luminal epithelium. On days 14 to 16, heifers received uterine infusions of the anti-estrogen ICI 182, 780, estradiol 17beta, IFN-tau or the saline control. On day 16, reproductive tracts were collected for analysis of integrin alphavbeta3 and ERalpha. Estrogen receptor alpha immunoreactivity was largely restricted to the luminal epithelium in control animals. Using anti-ERalpha recognizing the amino terminus, estrogen-treated animals showed reactivity in the stroma, shallow and deep glands and myometrium as is typical of estrus, whereas ICI 182, 870 treated heifers showed little or no reactivity. In contrast, carboxyl terminus-directed antibodies showed a widespread distribution of ERalpha with reactivity detected in the uterine epithelium, stroma and myometrium of both estrogen and ICI 182, 780 treated animals. Heifers treated with IFN-tau had low ERalpha reactivity overall. Control and IFN-tau treated heifers had lower intercaruncular stromal expression of integrin alphavbeta3 in comparison to estrogen and ICI 182, 780 treatments. Overall, the results suggest that on day 16 of the estrous cycle, estrogen effects on integrin alphavbeta3 are indirect and do not directly involve ERalpha in the luminal epithelium. During pregnancy, interferon-tau may block ERalpha in the luminal epithelium but likely does not rescue integrin alphavbeta3 expression.
Figures


Similar articles
-
Effects of the estrous cycle, pregnancy and interferon tau on expression of cyclooxygenase two (COX-2) in ovine endometrium.Reprod Biol Endocrinol. 2003 Aug 20;1:58. doi: 10.1186/1477-7827-1-58. Reprod Biol Endocrinol. 2003. PMID: 12956885 Free PMC article.
-
Effects of interferon-tau and progesterone on oestrogen-stimulated expression of receptors for oestrogen, progesterone and oxytocin in the endometrium of ovariectomized ewes.Reprod Fertil Dev. 1996;8(5):843-53. doi: 10.1071/rd9960843. Reprod Fertil Dev. 1996. PMID: 8876043
-
Ovine interferon-tau regulates expression of endometrial receptors for estrogen and oxytocin but not progesterone.Biol Reprod. 1995 Sep;53(3):732-45. doi: 10.1095/biolreprod53.3.732. Biol Reprod. 1995. PMID: 7578700
-
Ovine interferon-tau inhibits estrogen receptor up-regulation and estrogen-induced luteolysis in cyclic ewes.Endocrinology. 1995 Nov;136(11):4932-44. doi: 10.1210/endo.136.11.7588227. Endocrinology. 1995. PMID: 7588227
-
Interferon tau: a novel pregnancy recognition signal.Am J Reprod Immunol. 1997 Jun;37(6):412-20. doi: 10.1111/j.1600-0897.1997.tb00253.x. Am J Reprod Immunol. 1997. PMID: 9228295 Review.
Cited by
-
Antiproliferative Role of Natural and Semi-Synthetic Tocopherols on Colorectal Cancer Cells Overexpressing the Estrogen Receptor β.Int J Mol Sci. 2025 Mar 5;26(5):2305. doi: 10.3390/ijms26052305. Int J Mol Sci. 2025. PMID: 40076925 Free PMC article.
-
Immunohistochemical localization of integrin alpha V beta 3 and osteopontin suggests that they do not interact during embryo implantation in ruminants.Reprod Biol Endocrinol. 2004 Apr 28;2:19. doi: 10.1186/1477-7827-2-19. Reprod Biol Endocrinol. 2004. PMID: 15115551 Free PMC article.
-
Exosomes derived from placental trophoblast cells regulate endometrial epithelial receptivity in dairy cows during pregnancy.J Reprod Dev. 2022 Feb 18;68(1):21-29. doi: 10.1262/jrd.2021-077. Epub 2021 Oct 22. J Reprod Dev. 2022. PMID: 34690214 Free PMC article.
References
-
- Betteridge KJ, Eaglesome MD, Randall GC, Mitchell D. Collection, description and transfer of embryos from cattle 10–16 days after oestrus. J Reprod Fertil. 1980;59:205–16. - PubMed
-
- McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev. 1999;79:263–323. - PubMed
-
- Kimmins S, MacLaren LA. Cyclic modulation of integrin expression in bovine endometrium. Biol Reprod. 1999;61:1267–74. - PubMed
-
- Kimmins S, MacLaren LA. Oestrous cycle and pregnancy effects on the distribution of oestrogen and progesterone receptors in bovine endometrium. Placenta. 2001;22:742–8. - PubMed
-
- Hynes RO. Integrins: Bidirectional, allosteric signalling machines. Cell. 2002;110:673–687. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

