The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces
- PMID: 12756232
- PMCID: PMC2199363
- DOI: 10.1083/jcb.200302151
The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces
Abstract
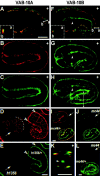
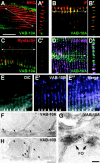
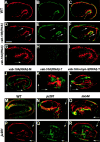
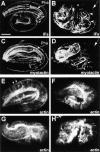
Morphogenesis of the Caenorhabditis elegans embryo is driven by actin microfilaments in the epidermis and by sarcomeres in body wall muscles. Both tissues are mechanically coupled, most likely through specialized attachment structures called fibrous organelles (FOs) that connect muscles to the cuticle across the epidermis. Here, we report the identification of new mutations in a gene known as vab-10, which lead to severe morphogenesis defects, and show that vab-10 corresponds to the C. elegans spectraplakin locus. Our analysis of vab-10 reveals novel insights into the role of this plakin subfamily. vab-10 generates isoforms related either to plectin (termed VAB-10A) or to microtubule actin cross-linking factor plakins (termed VAB-10B). Using specific antibodies and mutations, we show that VAB-10A and VAB-10B have distinct distributions and functions in the epidermis. Loss of VAB-10A impairs the integrity of FOs, leading to epidermal detachment from the cuticle and muscles, hence demonstrating that FOs are functionally and molecularly related to hemidesmosomes. We suggest that this isoform protects against forces external to the epidermis. In contrast, lack of VAB-10B leads to increased epidermal thickness during embryonic morphogenesis when epidermal cells change shape. We suggest that this isoform protects cells against tension that builds up within the epidermis.
Figures





References
-
- Baum, P.D., and G. Garriga. 1997. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron. 19:51–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

