Colocalization of synapsin and actin during synaptic vesicle recycling
- PMID: 12756235
- PMCID: PMC2199372
- DOI: 10.1083/jcb.200212140
Colocalization of synapsin and actin during synaptic vesicle recycling
Abstract
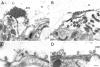
It has been hypothesized that in the mature nerve terminal, interactions between synapsin and actin regulate the clustering of synaptic vesicles and the availability of vesicles for release during synaptic activity. Here, we have used immunogold electron microscopy to examine the subcellular localization of actin and synapsin in the giant synapse in lamprey at different states of synaptic activity. In agreement with earlier observations, in synapses at rest, synapsin immunoreactivity was preferentially localized to a portion of the vesicle cluster distal to the active zone. During synaptic activity, however, synapsin was detected in the pool of vesicles proximal to the active zone. In addition, actin and synapsin were found colocalized in a dynamic filamentous cytomatrix at the sites of synaptic vesicle recycling, endocytic zones. Synapsin immunolabeling was not associated with clathrin-coated intermediates but was found on vesicles that appeared to be recycling back to the cluster. Disruption of synapsin function by microinjection of antisynapsin antibodies resulted in a prominent reduction of the cytomatrix at endocytic zones of active synapses. Our data suggest that in addition to its known function in clustering of vesicles in the reserve pool, synapsin migrates from the synaptic vesicle cluster and participates in the organization of the actin-rich cytomatrix in the endocytic zone during synaptic activity.
Figures






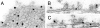
References
-
- Benfenati, F., F. Valtorta, M. Bahler, and P. Greengard. 1989. Synapsin I, a neuron-specific phosphoprotein interacting with small synaptic vesicles and F-actin. Cell Biol. Int. Rep. 13:1007–1021. - PubMed
-
- Benfenati, F., F. Valtorta, E. Chieregatti, and P. Greengard. 1992. Interaction of free and synaptic vesicle-bound synapsin I with F-actin. Neuron. 8:377–386. - PubMed
-
- Brodin, L., O. Shupliakov, V.A. Pieribone, J. Hellgren, and R.H. Hill. 1994. The reticulospinal glutamate synapse in lamprey: plasticity and presynaptic variability. J. Neurophysiol. 72:592–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

