Antigen-capturing cells can masquerade as memory B cells
- PMID: 12756262
- PMCID: PMC2193792
- DOI: 10.1084/jem.20020270
Antigen-capturing cells can masquerade as memory B cells
Abstract
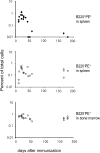
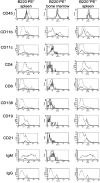
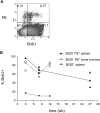
As well as classically defined switched immunoglobulin isotype-expressing B cells, memory B cells are now thought to include IgM-expressing cells and memory cells that lack B cell lineage markers, such as B220 or CD19. We set out to compare the relative importance of memory B cell subsets with an established flow cytometry method to identify antigen-specific cells. After immunization with PE, we could detect B220+ and, as reported previously, B220- antigen-binding cells (McHeyzer-Williams, L.J., M. Cool, and M.G. McHeyzer-Williams. 2001. J. Immunol. 167:1393-1405). The B220-PE+ cells bore few markers typical of B cells, but resembled myeloid cells. Further analysis of the antigen-binding characteristics of these cells showed that, upon immunization with two fluorescent proteins, the B220- cells could bind both. Furthermore, this subpopulation was detected in RAG1-/- mice after transfer of anti-PE mouse serum. These data strongly suggest that these cells capture serum Ig, via Fc receptors, and thus appear antigen-specific. Investigation of these antigen-capturing cells in a variety of knockout mice indicates that they bind monomeric IgG in an FcgammaR1 (CD64)-dependent manner. We find no evidence of a B220- memory B cell population that is not explicable by antigen-capturing cells, and warn that care must be taken when using antigen-specificity or surface IgG as an indicator of B cell memory.
Figures






References
-
- Schittek, B., and K. Rajewsky. 1990. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 346:749–751. - PubMed
-
- McHeyzer-Williams, M.G., G.J. Nossal, and P.A. Lalor. 1991. Molecular characterization of single memory B cells. Nature. 350:502–505. - PubMed
-
- Gray, D. 1993. Immunological memory. Annu. Rev. Immunol. 11:49–77. - PubMed
-
- Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

