Pneumocystis carinii pneumonia as a complication of bendamustine monotherapy in a patient with advanced progressive breast cancer
- PMID: 12756557
- PMCID: PMC12162061
- DOI: 10.1007/s00432-003-0441-y
Pneumocystis carinii pneumonia as a complication of bendamustine monotherapy in a patient with advanced progressive breast cancer
Abstract
Background: Bendamustine is an alkylator with anticipated antimetabolic activity. It has shown activity in malignant lymphoma, multiple myeloma, and breast cancer. Recognized side-effects are relatively mild with myelosuppression as the dose-limiting toxicity. The CD4/CD8 ratio may be reduced. To what extent the alteration of lymphocytes, especially CD4(+) lymphocytes, correlates with an increase in opportunistic infections cannot be definitively answered.
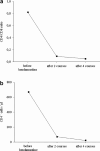
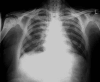
Case report: The patient, female, aged 48 years, was suffering from an advanced progressive breast cancer. After initial treatment with several chemotherapies, a cytotoxic therapy was initiated, with bendamustine (150 mg/m(2)) administered on two consecutive days and repeated every 4 weeks. After five courses, the patient developed Pneumocystis carinii pneumonia (PCP), disclosed in the bronchoalveolar lavage. While receiving bendamustine therapy, the CD4(+) and CD8(+) lymphocyte counts in the peripheral blood were determined by flow cytometry. The next-to-normal CD4/CD8 ratio before therapy (0,82) had decreased to 0,05 during the therapy mainly due to a decline of CD4(+) lymphocyte. The patient was seronegative for human immunodeficiency virus. In spite of high-dose intravenous trimethoprim/sulfamethoxazole and methylprednisolone application, the patient died of a respiratory failure 3 days after PCP was diagnosed.
Conclusion: Bendamustine is capable of inducing a reduction in CD4(+) lymphocyte counts causing a severe T-lymphocyte-mediated immunosuppression. Measuring CD4(+) lymphocyte counts may be helpful in determining the risk of PCP in patients treated with bendamustine.
Figures
Similar articles
-
Repeated administration of short infusions of bendamustine: a phase I study in patients with advanced progressive solid tumours.J Cancer Res Clin Oncol. 2000 Jan;126(1):41-7. doi: 10.1007/pl00008463. J Cancer Res Clin Oncol. 2000. PMID: 10641748 Free PMC article. Clinical Trial.
-
Phase-I/II study to evaluate dose limiting toxicity, maximum tolerated dose, and tolerability of bendamustine HCl in pre-treated patients with B-chronic lymphocytic leukaemia (Binet stages B and C) requiring therapy.J Cancer Res Clin Oncol. 2006 Feb;132(2):99-104. doi: 10.1007/s00432-005-0050-z. Epub 2005 Nov 15. J Cancer Res Clin Oncol. 2006. PMID: 16292542 Free PMC article. Clinical Trial.
-
Regression of brain metastases from breast carcinoma after chemotherapy with bendamustine.J Cancer Res Clin Oncol. 2002 Feb;128(2):111-3. doi: 10.1007/s00432-001-0303-4. Epub 2001 Nov 30. J Cancer Res Clin Oncol. 2002. PMID: 11862482 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Bendamustine for patients with indolent B cell lymphoid malignancies including chronic lymphocytic leukaemia.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD009045. doi: 10.1002/14651858.CD009045.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972131 Free PMC article.
Cited by
-
Routine use of bendamustine and rituximab combination therapy in consecutive patients with lymphoproliferative diseases: a survey from Tyrolean hospitals.Wien Klin Wochenschr. 2011 May;123(9-10):269-75. doi: 10.1007/s00508-011-1558-7. Epub 2011 Apr 13. Wien Klin Wochenschr. 2011. PMID: 21479652
-
Pneumocystis jiroveci prophylaxis in patients undergoing Bendamustine treatment: the need for a standardized protocol.Clin Case Rep. 2015 Apr;3(4):255-9. doi: 10.1002/ccr3.195. Epub 2015 Feb 9. Clin Case Rep. 2015. PMID: 25914820 Free PMC article.
-
Long-term outcome of progressive multifocal leukoencephalopathy with recombinant interleukin-2 treatment and an associated increase in the number of HPyV-2-specific T-cells: a case report.Ther Adv Hematol. 2023 Oct 9;14:20406207231201721. doi: 10.1177/20406207231201721. eCollection 2023. Ther Adv Hematol. 2023. PMID: 37822572 Free PMC article.
-
Increasing Pneumocystis pneumonia, England, UK, 2000-2010.Emerg Infect Dis. 2013 Mar;19(3):386-92. doi: 10.3201/eid1903.121151. Emerg Infect Dis. 2013. PMID: 23622345 Free PMC article.
-
Pneumocystis jiroveci Pneumonia in an Atypical Host.Clin Breast Cancer. 2012 Apr;12(2):138-41. doi: 10.1016/j.clbc.2011.10.003. Epub 2011 Dec 1. Clin Breast Cancer. 2012. PMID: 22133356 Free PMC article. No abstract available.
References
-
- Strumberg D, Harstrick A, Doll K, et al (1996) Bendamustine hydrochloride activity against doxorubicine-resistant human breast carcinoma cell lines. Anti Cancer Drugs 7:415–421 - PubMed
-
- Reck M, Haering B, Koschel G, et al (1998) Chemotherapie des fortgeschrittenen nicht-kleinzelligen und kleinzelligen Bronchialkarzinoms mit Bendamustin—Eine Phase-II-Studie. Pneumologie 52:571–574 - PubMed
-
- Rahn AN, Schilcher RB, Adamietz IA, et al (2001) Palliative Radiochemotherapie mit Bendamustin bei fortgeschrittenen Tumorrezidiven im HNO-Bereich. Strahlenther Onkol 177:189–194 - PubMed
-
- Bremer K, Roth W (1996) Bendamustine, a low-toxic nitrogen-mustard derivative with high efficacy in malignant lymphomas. Tumordiagn Ther 17:1–6
-
- Matthias M, Preiss R, Sohr R, Possinger K (1995) Pharmakokinetics of bendamustine in patients with malignant tumors. Proc Am Assoc Clin Oncol 14:458 [abstr]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

